Spectrotemporal processing in spectral tuning modules of cat primary auditory cortex
- PMID: 22384036
- PMCID: PMC3288040
- DOI: 10.1371/journal.pone.0031537
Spectrotemporal processing in spectral tuning modules of cat primary auditory cortex
Abstract
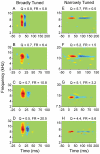
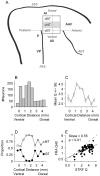
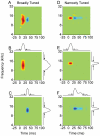
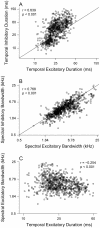
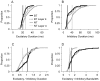
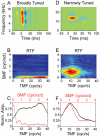
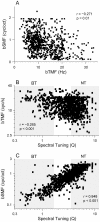
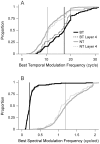
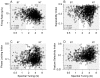
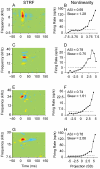
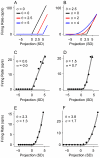
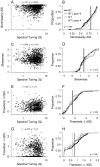
Spectral integration properties show topographical order in cat primary auditory cortex (AI). Along the iso-frequency domain, regions with predominantly narrowly tuned (NT) neurons are segregated from regions with more broadly tuned (BT) neurons, forming distinct processing modules. Despite their prominent spatial segregation, spectrotemporal processing has not been compared for these regions. We identified these NT and BT regions with broad-band ripple stimuli and characterized processing differences between them using both spectrotemporal receptive fields (STRFs) and nonlinear stimulus/firing rate transformations. The durations of STRF excitatory and inhibitory subfields were shorter and the best temporal modulation frequencies were higher for BT neurons than for NT neurons. For NT neurons, the bandwidth of excitatory and inhibitory subfields was matched, whereas for BT neurons it was not. Phase locking and feature selectivity were higher for NT neurons. Properties of the nonlinearities showed only slight differences across the bandwidth modules. These results indicate fundamental differences in spectrotemporal preferences--and thus distinct physiological functions--for neurons in BT and NT spectral integration modules. However, some global processing aspects, such as spectrotemporal interactions and nonlinear input/output behavior, appear to be similar for both neuronal subgroups. The findings suggest that spectral integration modules in AI differ in what specific stimulus aspects are processed, but they are similar in the manner in which stimulus information is processed.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

