Structural diversity in bacterial ribosomes: mycobacterial 70S ribosome structure reveals novel features
- PMID: 22384065
- PMCID: PMC3286452
- DOI: 10.1371/journal.pone.0031742
Structural diversity in bacterial ribosomes: mycobacterial 70S ribosome structure reveals novel features
Abstract
Here we present analysis of a 3D cryo-EM map of the 70S ribosome from Mycobacterium smegmatis, a saprophytic cousin of the etiological agent of tuberculosis in humans, Mycobacterium tuberculosis. In comparison with the 3D structures of other prokaryotic ribosomes, the density map of the M. smegmatis 70S ribosome reveals unique structural features and their relative orientations in the ribosome. Dramatic changes in the periphery due to additional rRNA segments and extra domains of some of the peripheral ribosomal proteins like S3, S5, S16, L17, L25, are evident. One of the most notable features appears in the large subunit near L1 stalk as a long helical structure next to helix 54 of the 23S rRNA. The sharp upper end of this structure is located in the vicinity of the mRNA exit channel. Although the M. smegmatis 70S ribosome possesses conserved core structure of bacterial ribosome, the new structural features, unveiled in this study, demonstrates diversity in the 3D architecture of bacterial ribosomes. We postulate that the prominent helical structure related to the 23S rRNA actively participates in the mechanisms of translation in mycobacteria.
Conflict of interest statement
Figures




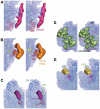

References
-
- Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature. 2009;461:1234–1242. - PubMed
-
- Baker R, Ridell M, Lind A, Ouchterlony O. Immunodiffusion studies of various structural preparations from mycobacterial cells. Int Arch Allergy Appl Immunol. 1979;59:328–336. - PubMed
-
- Lonnroth I, Ridell M. Analyses of ribosomal proteins from mycobacteria with particular reference to the beta antigen. FEMS Microbiology Letters. 1985;27:203–207.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

