Comparative gene expression profiling of P. falciparum malaria parasites exposed to three different histone deacetylase inhibitors
- PMID: 22384084
- PMCID: PMC3288058
- DOI: 10.1371/journal.pone.0031847
Comparative gene expression profiling of P. falciparum malaria parasites exposed to three different histone deacetylase inhibitors
Abstract
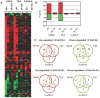
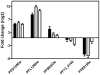
Histone deacetylase (HDAC) inhibitors are being intensively pursued as potential new drugs for a range of diseases, including malaria. HDAC inhibitors are also important tools for the study of epigenetic mechanisms, transcriptional control, and other important cellular processes. In this study the effects of three structurally related antimalarial HDAC inhibitors on P. falciparum malaria parasite gene expression were compared. The three hydroxamate-based compounds, trichostatin A (TSA), suberoylanilide hydroxamic acid (SAHA; Vorinostat®) and a 2-aminosuberic acid derivative (2-ASA-9), all caused profound transcriptional effects, with ~2-21% of genes having >2-fold altered expression following 2 h exposure to the compounds. Only two genes, alpha tubulin II and a hydrolase, were up-regulated by all three compounds after 2 h exposure in all biological replicates examined. The transcriptional changes observed after 2 h exposure to HDAC inhibitors were found to be largely transitory, with only 1-5% of genes being regulated after removing the compounds and culturing for a further 2 h. Despite some structural similarity, the three inhibitors caused quite diverse transcriptional effects, possibly reflecting subtle differences in mode of action or cellular distribution. This dataset represents an important contribution to our understanding of how HDAC inhibitors act on malaria parasites and identifies alpha tubulin II as a potential transcriptional marker of HDAC inhibition in malaria parasites that may be able to be exploited for future development of HDAC inhibitors as new antimalarial agents.
Conflict of interest statement
Figures



References
-
- Andrews KT, Tran TN, Wheatley NC, Fairlie DP. Targeting histone deacetylase inhibitors for anti-malarial therapy. Curr Top Med Chem. 2009;9:292–308. - PubMed
-
- Andrews KT, Haque A, Jones MK. HDAC inhibitors in parasitic diseases. Immunol Cell Biol. 2012;90:66–77. - PubMed
-
- Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. - PubMed
-
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, et al. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121:13–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

