Interleukin-7 links T lymphocyte and intestinal epithelial cell homeostasis
- PMID: 22384106
- PMCID: PMC3288069
- DOI: 10.1371/journal.pone.0031939
Interleukin-7 links T lymphocyte and intestinal epithelial cell homeostasis
Abstract
Interleukin-7 (IL-7) is a major survival factor for mature T cells. Therefore, the degree of IL-7 availability determines the size of the peripheral T cell pool and regulates T cell homeostasis. Here we provide evidence that IL-7 also regulates the homeostasis of intestinal epithelial cells (IEC), colon function and the composition of the commensal microflora. In the colon of T cell-deficient, lymphopenic mice, IL-7-producing IEC accumulate. IEC hyperplasia can be blocked by IL-7-consuming T cells or the inactivation of the IL-7/IL-7R signaling pathway. However, the blockade of the IL-7/IL-7R signaling pathway renders T cell-deficient mice more sensitive to chemically-induced IEC damage and subsequent colitis. In summary, our data demonstrate that IL-7 promotes IEC hyperplasia under lymphopenic conditions. Under non-lymphopenic conditions, however, T cells consume IL-7 thereby limiting IEC expansion and survival. Hence, the degree of IL-7 availability regulates both, T cell and IEC homeostasis.
Conflict of interest statement
Figures

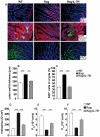
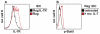


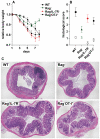
References
-
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. - PubMed
-
- Khaled AR, Durum SK. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat Rev Immunol. 2002;2:817–830. - PubMed
-
- Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. - PubMed
-
- Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

