Roles of MAPK and spindle assembly checkpoint in spontaneous activation and MIII arrest of rat oocytes
- PMID: 22384134
- PMCID: PMC3288063
- DOI: 10.1371/journal.pone.0032044
Roles of MAPK and spindle assembly checkpoint in spontaneous activation and MIII arrest of rat oocytes
Abstract
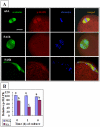
Rat oocytes are well known to undergo spontaneous activation (SA) after leaving the oviduct, but the SA is abortive with oocytes being arrested in metaphase III (MIII) instead of forming pronuclei. This study was designed to investigate the mechanism causing SA and MIII arrest. Whereas few oocytes collected from SD rats at 13 h after hCG injection that showed 100% of mitogen-activated protein kinase (MAPK) activities activated spontaneously, all oocytes recovered 19 h post hCG with MAPK decreased to below 75% underwent SA during in vitro culture. During SA, MAPK first declined to below 45% and then increased again to 80%; the maturation-promoting factor (MPF) activity fluctuated similarly but always began to change ahead of the MAPK activity. In SA oocytes with 75% of MAPK activities, microtubules were disturbed with irregularly pulled chromosomes dispersed over the spindle and the spindle assembly checkpoint (SAC) was activated. When MAPK decreased to 45%, the spindle disintegrated and chromosomes surrounded by microtubules were scattered in the ooplasm. SA oocytes entered MIII and formed several spindle-like structures by 6 h of culture when the MAPK activity re-increased to above 80%. While SA oocytes showed one Ca(2+) rise, Sr(2+)-activated oocytes showed several. Together, the results suggested that SA stimuli triggered SA in rat oocytes by inducing a premature MAPK inactivation, which led to disturbance of spindle microtubules. The microtubule disturbance impaired pulling of chromosomes to the spindle poles, caused spindle disintegration and activated SAC. The increased SAC activity reactivated MPF and thus MAPK, leading to MIII arrest.
Conflict of interest statement
Figures





Similar articles
-
Differential inactivation of maturation-promoting factor and mitogen-activated protein kinase following parthenogenetic activation of bovine oocytes.Biol Reprod. 1998 Sep;59(3):537-45. doi: 10.1095/biolreprod59.3.537. Biol Reprod. 1998. PMID: 9716551
-
Mitogen activated protein kinase plays a significant role in metaphase II arrest, spindle morphology, and maintenance of maturation promoting factor activity in bovine oocytes.Mol Reprod Dev. 2001 May;59(1):106-14. doi: 10.1002/mrd.1012. Mol Reprod Dev. 2001. PMID: 11335952
-
Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice.Biol Reprod. 2012 Mar 22;86(3):83. doi: 10.1095/biolreprod.111.095281. Print 2012 Mar. Biol Reprod. 2012. PMID: 22133696
-
The pathway of MAP kinase mediation of CSF arrest in Xenopus oocytes.Biol Cell. 2001 Sep;93(1-2):27-33. doi: 10.1016/s0248-4900(01)01127-3. Biol Cell. 2001. PMID: 11730319 Review.
-
Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals.Biol Reprod. 2004 Mar;70(3):535-47. doi: 10.1095/biolreprod.103.022830. Epub 2003 Nov 12. Biol Reprod. 2004. PMID: 14613897 Review.
Cited by
-
Role of Na+/Ca2+ exchanger (NCX) in modulating postovulatory aging of mouse and rat oocytes.PLoS One. 2014 Apr 2;9(4):e93446. doi: 10.1371/journal.pone.0093446. eCollection 2014. PLoS One. 2014. PMID: 24695407 Free PMC article.
-
Effect of the time interval between oocyte retrieval and ICSI on embryo development and reproductive outcomes: a systematic review.Reprod Biol Endocrinol. 2021 Mar 1;19(1):34. doi: 10.1186/s12958-021-00717-0. Reprod Biol Endocrinol. 2021. PMID: 33648503 Free PMC article.
-
Oocyte Spontaneous Activation: An Overlooked Cellular Event That Impairs Female Fertility in Mammals.Front Cell Dev Biol. 2021 Mar 8;9:648057. doi: 10.3389/fcell.2021.648057. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33763428 Free PMC article. Review.
-
Expression profiles and function analysis of microRNAs in postovulatory aging mouse oocytes.Aging (Albany NY). 2017 Apr;9(4):1186-1201. doi: 10.18632/aging.101219. Aging (Albany NY). 2017. PMID: 28394765 Free PMC article.
-
The signaling pathways by which the Fas/FasL system accelerates oocyte aging.Aging (Albany NY). 2016 Feb;8(2):291-303. doi: 10.18632/aging.100893. Aging (Albany NY). 2016. PMID: 26869336 Free PMC article.
References
-
- Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet. 2002;3:33–42. - PubMed
-
- Zan Y, Haag JD, Chen KS, Shepel LA, Wigington D, et al. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol. 2003;21:645–651. - PubMed
-
- Hayes E, Galea S, Verkuylen A, Pera M, Morrison J, et al. Nuclear transfer of adult and genetically modified fetal cells of the rat. Physiol Genomics. 2001;5:193–204. - PubMed
-
- Hirabayashi M, Kato M, Ishikawa A, Hochi S. Factors influencing chromosome condensation and development of cloned rat embryos. Cloning Stem Cells. 2003;5:35–42. - PubMed
-
- Iannaccone P, Taborn G, Garton R. Preimplantation and postimplantation development of rat embryos cloned with cumulus cells and fibroblasts. Zygote. 2001;9:135–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

