Sprouty4 is an endogenous negative modulator of TrkA signaling and neuronal differentiation induced by NGF
- PMID: 22384148
- PMCID: PMC3285629
- DOI: 10.1371/journal.pone.0032087
Sprouty4 is an endogenous negative modulator of TrkA signaling and neuronal differentiation induced by NGF
Abstract
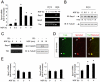
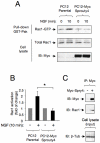
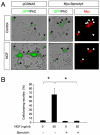
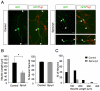
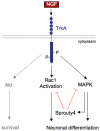
The Sprouty (Spry) family of proteins represents endogenous regulators of downstream signaling pathways induced by receptor tyrosine kinases (RTKs). Using real time PCR, we detect a significant increase in the expression of Spry4 mRNA in response to NGF, indicating that Spry4 could modulate intracellular signaling pathways and biological processes induced by NGF and its receptor TrkA. In this work, we demonstrate that overexpression of wild-type Spry4 causes a significant reduction in MAPK and Rac1 activation and neurite outgrowth induced by NGF. At molecular level, our findings indicate that ectopic expression of a mutated form of Spry4 (Y53A), in which a conserved tyrosine residue was replaced, fail to block both TrkA-mediated Erk/MAPK activation and neurite outgrowth induced by NGF, suggesting that an intact tyrosine 53 site is required for the inhibitory effect of Spry4 on NGF signaling. Downregulation of Spry4 using small interference RNA knockdown experiments potentiates PC12 cell differentiation and MAPK activation in response to NGF. Together, these findings establish a new physiological mechanism through which Spry4 regulates neurite outgrowth reducing not only the MAPK pathway but also restricting Rac1 activation in response to NGF.
Conflict of interest statement
Figures



References
-
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. - PubMed
-
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. - PubMed
-
- Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–135. - PubMed
-
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

