Longitudinal tracking of human fetal cells labeled with super paramagnetic iron oxide nanoparticles in the brain of mice with motor neuron disease
- PMID: 22384217
- PMCID: PMC3288077
- DOI: 10.1371/journal.pone.0032326
Longitudinal tracking of human fetal cells labeled with super paramagnetic iron oxide nanoparticles in the brain of mice with motor neuron disease
Abstract
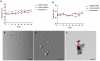
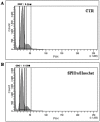
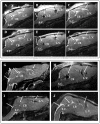
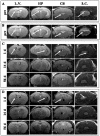
Stem Cell (SC) therapy is one of the most promising approaches for the treatment of Amyotrophic Lateral Sclerosis (ALS). Here we employed Super Paramagnetic Iron Oxide nanoparticles (SPIOn) and Hoechst 33258 to track human Amniotic Fluid Cells (hAFCs) after transplantation in the lateral ventricles of wobbler (a murine model of ALS) and healthy mice. By in vitro, in vivo and ex vivo approaches we found that: 1) the main physical parameters of SPIOn were maintained over time; 2) hAFCs efficiently internalized SPIOn into the cytoplasm while Hoechst 33258 labeled nuclei; 3) SPIOn internalization did not alter survival, cell cycle, proliferation, metabolism and phenotype of hAFCs; 4) after transplantation hAFCs rapidly spread to the whole ventricular system, but did not migrate into the brain parenchyma; 5) hAFCs survived for a long time in the ventricles of both wobbler and healthy mice; 6) the transplantation of double-labeled hAFCs did not influence mice survival.
Conflict of interest statement
Figures



Similar articles
-
Superparamagnetic iron oxide nanoparticles as a tool to track mouse neural stem cells in vivo.Mol Biol Rep. 2019 Feb;46(1):191-198. doi: 10.1007/s11033-018-4460-9. Epub 2018 Nov 12. Mol Biol Rep. 2019. PMID: 30421128
-
Dose dependent side effect of superparamagnetic iron oxide nanoparticle labeling on cell motility in two fetal stem cell populations.PLoS One. 2013 Nov 7;8(11):e78435. doi: 10.1371/journal.pone.0078435. eCollection 2013. PLoS One. 2013. PMID: 24244310 Free PMC article. Clinical Trial.
-
Neurorescue effects and stem properties of chorionic villi and amniotic progenitor cells.Neuroscience. 2013 Mar 27;234:158-72. doi: 10.1016/j.neuroscience.2012.12.038. Epub 2013 Jan 3. Neuroscience. 2013. PMID: 23291343
-
Tracking stem cells with superparamagnetic iron oxide nanoparticles: perspectives and considerations.Int J Nanomedicine. 2017 Jan 25;12:779-793. doi: 10.2147/IJN.S126530. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28182122 Free PMC article. Review.
-
Dual contrast magnetic resonance imaging tracking of iron-labeled cells in vivo.Cytotherapy. 2010 Nov;12(7):859-69. doi: 10.3109/14653241003587652. Cytotherapy. 2010. PMID: 20184501 Review.
Cited by
-
Internalization of nanopolymeric tracers does not alter characteristics of placental cells.J Cell Mol Med. 2016 Jun;20(6):1036-48. doi: 10.1111/jcmm.12820. Epub 2016 Mar 14. J Cell Mol Med. 2016. PMID: 26987908 Free PMC article.
-
Non-Temperature Induced Effects of Magnetized Iron Oxide Nanoparticles in Alternating Magnetic Field in Cancer Cells.PLoS One. 2016 May 31;11(5):e0156294. doi: 10.1371/journal.pone.0156294. eCollection 2016. PLoS One. 2016. PMID: 27244470 Free PMC article.
-
Toxicological Aspects of Iron Oxide Nanoparticles.Adv Exp Med Biol. 2022;1357:303-350. doi: 10.1007/978-3-030-88071-2_13. Adv Exp Med Biol. 2022. PMID: 35583650
-
Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: clinical needs, biomaterials, and imaging technologies.NPJ Regen Med. 2018 Apr 4;3:8. doi: 10.1038/s41536-018-0046-3. eCollection 2018. NPJ Regen Med. 2018. PMID: 29644098 Free PMC article. Review.
-
Neuroprotective effects of GDNF-expressing human amniotic fluid cells.Stem Cell Rev Rep. 2014 Apr;10(2):251-68. doi: 10.1007/s12015-013-9484-x. Stem Cell Rev Rep. 2014. PMID: 24415130
References
-
- Silani V, Calzarossa C, Cova L, Ticozzi N. Stem cells in amyotrophic lateral sclerosis: motor neuron protection or replacement? CNS Neurol Disord Drug Targets. 2010;9:314–324. - PubMed
-
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. - PubMed
-
- Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39:2501–2510. - PubMed
-
- Hedlund E, Hefferan MP, Marsala M, Isacson O. Cell therapy and stem cells in animal models of motor neuron disorders. Eur J Neurosci. 2007;26:1721–1737. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

