Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair
- PMID: 22384219
- PMCID: PMC3284560
- DOI: 10.1371/journal.pone.0032333
Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair
Abstract
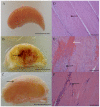
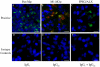
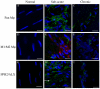
Macrophages (Mφ) orchestrate inflammatory and reparatory processes in injured connective tissues but their role during different phases of tendon healing is not known. We investigated the contribution of different Mφ subsets in an equine model of naturally occurring tendon injury. Post mortem tissues were harvested from normal (uninjured), sub-acute (3-6 weeks post injury) and chronically injured (>3 months post injury) superficial digital flexor tendons. To determine if inflammation was present in injured tendons, Mφ sub-populations were quantified based on surface antigen expression of CD172a (pan Mφ), CD14(high)CD206(low) (pro-inflammatory M1Mφ), and CD206(high) (anti-inflammatory M2Mφ) to assess potential polarised phenotypes. In addition, the Lipoxin A(4) receptor (FPR2/ALX) was used as marker for resolving inflammation. Normal tendons were negative for both Mφ and FPR2/ALX. In contrast, M1Mφ predominated in sub-acute injury, whereas a potential phenotype-switch to M2Mφ polarity was seen in chronic injury. Furthermore, FPR2/ALX expression by tenocytes was significantly upregulated in sub-acute but not chronic injury. Expression of the FPR2/ALX ligand Annexin A1 was also significantly increased in sub-acute and chronic injuries in contrast to low level expression in normal tendons. The combination of reduced FPR2/ALX expression and persistence of the M2Mφ phenotype in chronic injury suggests a potential mechanism for incomplete resolution of inflammation after tendon injury. To investigate the effect of pro-inflammatory mediators on lipoxin A(4) (LXA(4)) production and FPR2/ALX expression in vitro, normal tendon explants were stimulated with interleukin-1 beta and prostaglandin E(2). Stimulation with either mediator induced LXA(4) release and maximal upregulation of FPR2/ALX expression after 72 hours. Taken together, our data suggests that although tenocytes are capable of mounting a protective mechanism to counteract inflammatory stimuli, this appears to be of insufficient duration and magnitude in natural tendon injury, which may potentiate chronic inflammation and fibrotic repair, as indicated by the presence of M2Mφ.
Conflict of interest statement
Figures






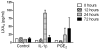
Similar articles
-
Inflamm-aging and arachadonic acid metabolite differences with stage of tendon disease.PLoS One. 2012;7(11):e48978. doi: 10.1371/journal.pone.0048978. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155437 Free PMC article.
-
Lack of resolution sensor drives age-related cardiometabolic and cardiorenal defects and impedes inflammation-resolution in heart failure.Mol Metab. 2020 Jan;31:138-149. doi: 10.1016/j.molmet.2019.10.008. Epub 2019 Nov 16. Mol Metab. 2020. PMID: 31918915 Free PMC article.
-
FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis.FASEB J. 2010 Nov;24(11):4240-9. doi: 10.1096/fj.10-159913. Epub 2010 Jun 22. FASEB J. 2010. PMID: 20570963 Free PMC article.
-
Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways.Pharmacol Ther. 2013 Dec;140(3):280-9. doi: 10.1016/j.pharmthera.2013.07.007. Epub 2013 Jul 21. Pharmacol Ther. 2013. PMID: 23880288 Review.
-
Lipoxin-mediated signaling: ALX/FPR2 interaction and beyond.Pharmacol Res. 2023 Nov;197:106982. doi: 10.1016/j.phrs.2023.106982. Epub 2023 Nov 3. Pharmacol Res. 2023. PMID: 37925045 Review.
Cited by
-
Species variations in tenocytes' response to inflammation require careful selection of animal models for tendon research.Sci Rep. 2021 Jun 14;11(1):12451. doi: 10.1038/s41598-021-91914-9. Sci Rep. 2021. PMID: 34127759 Free PMC article.
-
Inflamm-aging and arachadonic acid metabolite differences with stage of tendon disease.PLoS One. 2012;7(11):e48978. doi: 10.1371/journal.pone.0048978. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155437 Free PMC article.
-
Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation.J Biol Chem. 2014 Sep 12;289(37):25867-78. doi: 10.1074/jbc.M114.566554. Epub 2014 Jul 30. J Biol Chem. 2014. PMID: 25077967 Free PMC article.
-
CD146 Delineates an Interfascicular Cell Sub-Population in Tendon That Is Recruited during Injury through Its Ligand Laminin-α4.Int J Mol Sci. 2021 Sep 8;22(18):9729. doi: 10.3390/ijms22189729. Int J Mol Sci. 2021. PMID: 34575887 Free PMC article.
-
Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts.Am J Sports Med. 2016 Aug;44(8):1931-40. doi: 10.1177/0363546516637176. Epub 2016 Jul 8. Am J Sports Med. 2016. PMID: 27400714 Free PMC article.
References
-
- Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–135. - PubMed
-
- Avella CS, Ely ER, Verheyen KL, Price JS, Wood JL, et al. Ultrasonographic assessment of the superficial digital flexor tendons of National Hunt racehorses in training over two racing seasons. Equine Vet J. 2009;41:449–454. - PubMed
-
- Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, et al. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. - PubMed
-
- Jozsa L, Reffy A, Kannus P, Demel S, Elek E. Pathological alterations in human tendons. Arch Orthop Trauma Surg. 1990;110:15–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

