Surfactant protein-A suppresses eosinophil-mediated killing of Mycoplasma pneumoniae in allergic lungs
- PMID: 22384248
- PMCID: PMC3285686
- DOI: 10.1371/journal.pone.0032436
Surfactant protein-A suppresses eosinophil-mediated killing of Mycoplasma pneumoniae in allergic lungs
Abstract
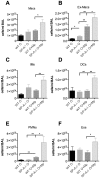
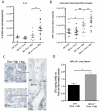
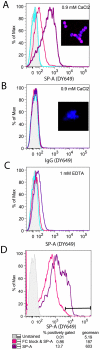
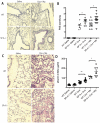
Surfactant protein-A (SP-A) has well-established functions in reducing bacterial and viral infections but its role in chronic lung diseases such as asthma is unclear. Mycoplasma pneumoniae (Mp) frequently colonizes the airways of chronic asthmatics and is thought to contribute to exacerbations of asthma. Our lab has previously reported that during Mp infection of non-allergic airways, SP-A aides in maintaining airway homeostasis by inhibiting an overzealous TNF-alpha mediated response and, in allergic mice, SP-A regulates eosinophilic infiltration and inflammation of the airway. In the current study, we used an in vivo model with wild type (WT) and SP-A(-/-) allergic mice challenged with the model antigen ovalbumin (Ova) that were concurrently infected with Mp (Ova+Mp) to test the hypothesis that SP-A ameliorates Mp-induced stimulation of eosinophils. Thus, SP-A could protect allergic airways from injury due to release of eosinophil inflammatory products. SP-A deficient mice exhibit significant increases in inflammatory cells, mucus production and lung damage during concurrent allergic airway disease and infection (Ova+Mp) as compared to the WT mice of the same treatment group. In contrast, SP-A deficient mice have significantly decreased Mp burden compared to WT mice. The eosinophil specific factor, eosinophil peroxidase (EPO), which has been implicated in pathogen killing and also in epithelial dysfunction due to oxidative damage of resident lung proteins, is enhanced in samples from allergic/infected SP-A(-/-) mice as compared to WT mice. In vitro experiments using purified eosinophils and human SP-A suggest that SP-A limits the release of EPO from Mp-stimulated eosinophils thereby reducing their killing capacity. These findings are the first to demonstrate that although SP-A interferes with eosinophil-mediated biologic clearance of Mp by mediating the interaction of Mp with eosinophils, SP-A simultaneously benefits the airway by limiting inflammation and damage.
Conflict of interest statement
Figures




Similar articles
-
SP-A preserves airway homeostasis during Mycoplasma pneumoniae infection in mice.J Immunol. 2009 Jun 15;182(12):7818-27. doi: 10.4049/jimmunol.0900452. J Immunol. 2009. PMID: 19494306 Free PMC article.
-
Mast cell TNF receptors regulate responses to Mycoplasma pneumoniae in surfactant protein A (SP-A)-/- mice.J Allergy Clin Immunol. 2012 Jul;130(1):205-14.e2. doi: 10.1016/j.jaci.2012.03.002. Epub 2012 Apr 12. J Allergy Clin Immunol. 2012. PMID: 22502799 Free PMC article.
-
Genetic variation in SP-A2 leads to differential binding to Mycoplasma pneumoniae membranes and regulation of host responses.J Immunol. 2015 Jun 15;194(12):6123-32. doi: 10.4049/jimmunol.1500104. Epub 2015 May 8. J Immunol. 2015. PMID: 25957169 Free PMC article.
-
Linking surfactant protein SP-D and IL-13: implications in asthma and allergy.Mol Immunol. 2013 May;54(1):98-107. doi: 10.1016/j.molimm.2012.10.039. Epub 2012 Dec 7. Mol Immunol. 2013. PMID: 23220073 Review.
-
Eosinophil-associated lung diseases. A cry for surfactant proteins A and D help?Am J Respir Cell Mol Biol. 2014 Nov;51(5):604-14. doi: 10.1165/rcmb.2014-0095TR. Am J Respir Cell Mol Biol. 2014. PMID: 24960334 Free PMC article. Review.
Cited by
-
Granulocyte Macrophage Colony-Stimulating Factor-Activated Eosinophils Promote Interleukin-23 Driven Chronic Colitis.Immunity. 2015 Jul 21;43(1):187-99. doi: 10.1016/j.immuni.2015.07.008. Immunity. 2015. PMID: 26200014 Free PMC article.
-
Club Cell Secretory Protein Deficiency Leads to Altered Lung Function.Am J Respir Crit Care Med. 2019 Feb 1;199(3):302-312. doi: 10.1164/rccm.201807-1345OC. Am J Respir Crit Care Med. 2019. PMID: 30543455 Free PMC article.
-
Surfactant protein D attenuates sub-epithelial fibrosis in allergic airways disease through TGF-β.Respir Res. 2014 Nov 29;15(1):143. doi: 10.1186/s12931-014-0143-9. Respir Res. 2014. PMID: 25472740 Free PMC article.
-
IGF1R as a Potential Pharmacological Target in Allergic Asthma.Biomedicines. 2021 Jul 29;9(8):912. doi: 10.3390/biomedicines9080912. Biomedicines. 2021. PMID: 34440118 Free PMC article.
-
miR-143-3p impacts on pulmonary inflammatory factors and cell apoptosis in mice with mycoplasmal pneumonia by regulating TLR4/MyD88/NF-κB pathway.Biosci Rep. 2020 Jul 31;40(7):BSR20193419. doi: 10.1042/BSR20193419. Biosci Rep. 2020. PMID: 32597476 Free PMC article.
References
-
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. - PubMed
-
- Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990;85:422–436. - PubMed
-
- American Academy of Allergy A, and Immunology. Asthma Stastics. 1996–2011. www.aaaai.org/media/statistics/asthma-statistics.asp. Accessed 2012 Feb 1.
-
- Prevention CfDCa. Asthma. 2009–2010. www.cdc.gov/nchs/fastats/asthma.htm. Accessed 2012 Feb 1.
-
- Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

