Mechanisms of loss of functions of human angiogenin variants implicated in amyotrophic lateral sclerosis
- PMID: 22384259
- PMCID: PMC3288110
- DOI: 10.1371/journal.pone.0032479
Mechanisms of loss of functions of human angiogenin variants implicated in amyotrophic lateral sclerosis
Abstract
Background: Mutations in the coding region of angiogenin (ANG) gene have been found in patients suffering from Amyotrophic Lateral Sclerosis (ALS). Neurodegeneration results from the loss of angiogenic ability of ANG (protein coded by ANG). In this work, we performed extensive molecular dynamics (MD) simulations of wild-type ANG and disease associated ANG variants to elucidate the mechanism behind the loss of ribonucleolytic activity and nuclear translocation activity, functions needed for angiogenesis.
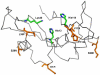
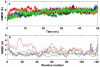
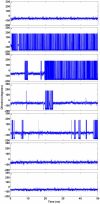
Methodology/principal findings: MD simulations were carried out to study the structural and dynamic differences in the catalytic site and nuclear localization signal residues between WT-ANG (Wild-type ANG) and six mutants. Variants K17I, S28N, P112L and V113I have confirmed association with ALS, while T195C and A238G single nucleotide polymorphisms (SNPs) encoding L35P and K60E mutants respectively, have not been associated with ALS. Our results show that loss of ribonucleolytic activity in K17I is caused by conformational switching of the catalytic residue His114 by 99°. The loss of nuclear translocation activity of S28N and P112L is caused by changes in the folding of the residues (31)RRR(33) that result in the reduction in solvent accessible surface area (SASA). Consequently, we predict that V113I will exhibit loss of angiogenic properties by loss of nuclear translocation activity and L35P by loss of both ribonucleolytic activity and nuclear translocation activity. No functional loss was inferred for K60E. The MD simulation results were supported by hydrogen bond interaction analyses and molecular docking studies.
Conclusions/significance: Conformational switching of catalytic residue His114 seems to be the mechanism causing loss of ribonucleolytic activity and reduction in SASA of nuclear localization signal residues (31)RRR(33) results in loss of nuclear translocation activity in ANG mutants. Therefore, we predict that L35P mutant, would exhibit loss of angiogenic functions, and hence would correlate with ALS while K60E would not show any loss.
Conflict of interest statement
Figures










References
-
- Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci. 2010;31:2247–2265. - PubMed
-
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

