Preventing phosphorylation of sterol regulatory element-binding protein 1a by MAP-kinases protects mice from fatty liver and visceral obesity
- PMID: 22384276
- PMCID: PMC3287979
- DOI: 10.1371/journal.pone.0032609
Preventing phosphorylation of sterol regulatory element-binding protein 1a by MAP-kinases protects mice from fatty liver and visceral obesity
Abstract
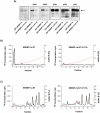
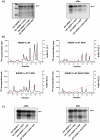
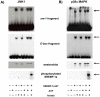
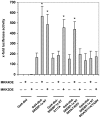
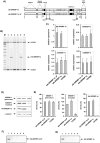
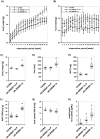
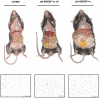
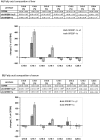
The transcription factor sterol regulatory element binding protein (SREBP)-1a plays a pivotal role in lipid metabolism. Using the SREBP-1a expressing human hepatoma cell line HepG2 we have shown previously that human SREBP-1a is phosphorylated at serine 117 by ERK-mitogen-activated protein kinases (MAPK). Using a combination of cell biology and protein chemistry approach we show that SREBP-1a is also target of other MAPK-families, i.e. c-JUN N-terminal protein kinases (JNK) or p38 stress activated MAP kinases. Serine 117 is also the major phosphorylation site in SREBP-1a for JNK. In contrast to that the major phosphorylation sites of p38 MAPK family are serine 63 and threonine 426. Functional analyses reveal that phosphorylation of SREBP-1a does not alter protein/DNA interaction. The identified phosphorylation sites are specific for both kinase families also in cellular context. To provide direct evidence that phosphorylation of SREBP-1a is a regulatory principle of biological and clinical relevance, we generated transgenic mice expressing mature transcriptionally active N-terminal domain of human SREBP-1a variant lacking all identified phosphorylaton sites designed as alb-SREBP-1aΔP and wild type SREBP-1a designed as alb-SREBP-1a liver specific under control of the albumin promoter and a liver specific enhancer. In contrast to alb-SREBP-1a mice the phosphorylation-deficient mice develop no enlarged fatty livers under normocaloric conditions. Phenotypical examination reveales a massive accumulation of adipose tissue in alb-SREBP-1a but not in the phosphorylation deficient alb-SREBP-1aΔP mice. Moreover, preventing phosphorylation of SREBP-1a protects mice also from dyslipidemia. In conclusion, phosphorylation of SREBP-1a by ERK, JNK and p38 MAPK-families resembles a biological principle and plays a significant role, in vivo.
Conflict of interest statement
Figures



Similar articles
-
Phosphorylation of sterol regulatory element-binding protein (SREBP)-1c by p38 kinases, ERK and JNK influences lipid metabolism and the secretome of human liver cell line HepG2.Arch Physiol Biochem. 2014 Dec;120(5):216-27. doi: 10.3109/13813455.2014.973418. Arch Physiol Biochem. 2014. PMID: 25353341
-
Inactivation of SREBP-1a Phosphorylation Prevents Fatty Liver Disease in Mice: Identification of Related Signaling Pathways by Gene Expression Profiles in Liver and Proteomes of Peroxisomes.Int J Mol Sci. 2018 Mar 25;19(4):980. doi: 10.3390/ijms19040980. Int J Mol Sci. 2018. PMID: 29587401 Free PMC article.
-
Lipid accumulation stimulates the cap-independent translation of SREBP-1a mRNA by promoting hnRNP A1 binding to its 5'-UTR in a cellular model of hepatic steatosis.Biochim Biophys Acta. 2016 May;1861(5):471-81. doi: 10.1016/j.bbalip.2016.02.003. Epub 2016 Feb 8. Biochim Biophys Acta. 2016. PMID: 26869449
-
Liver-specific expression of transcriptionally active SREBP-1c is associated with fatty liver and increased visceral fat mass.PLoS One. 2012;7(2):e31812. doi: 10.1371/journal.pone.0031812. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363740 Free PMC article.
-
Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism.Vitam Horm. 2002;65:167-94. doi: 10.1016/s0083-6729(02)65064-2. Vitam Horm. 2002. PMID: 12481547 Review.
Cited by
-
Expression profile of mitrogen-activated protein kinase (MAPK) signaling genes in the skeletal muscle & liver of rat with type 2 diabetes: role in disease pathology.Indian J Med Res. 2014 Dec;140(6):744-55. Indian J Med Res. 2014. PMID: 25758573 Free PMC article.
-
MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice.Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. Nat Commun. 2013. PMID: 24300912 Free PMC article.
-
Adipokinome Signatures in Obese Mouse Models Reflect Adipose Tissue Health and Are Associated with Serum Lipid Composition.Int J Mol Sci. 2019 May 24;20(10):2559. doi: 10.3390/ijms20102559. Int J Mol Sci. 2019. PMID: 31137678 Free PMC article.
-
CB1 blockade potentiates down-regulation of lipogenic gene expression in perirenal adipose tissue in high carbohydrate diet-induced obesity.PLoS One. 2014 Feb 25;9(2):e90016. doi: 10.1371/journal.pone.0090016. eCollection 2014. PLoS One. 2014. PMID: 24587189 Free PMC article.
-
[SREBP-1 and fatty liver. Clinical relevance for diabetes, obesity, dyslipidemia and atherosclerosis].Herz. 2012 May;37(3):273-8. doi: 10.1007/s00059-012-3608-y. Herz. 2012. PMID: 22453913 German.
References
-
- Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

