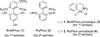
Palladium-Catalyzed Coupling of Functionalized Primary and Secondary Amines with Aryl and Heteroaryl Halides: Two Ligands Suffice in Most Cases
- PMID: 22384311
- PMCID: PMC3289414
- DOI: 10.1039/C0SC00330A
Palladium-Catalyzed Coupling of Functionalized Primary and Secondary Amines with Aryl and Heteroaryl Halides: Two Ligands Suffice in Most Cases
Abstract
We report our studies on the use of two catalyst systems, based on the ligands BrettPhos (1) and RuPhos (2), which provide the widest scope for Pd-catalyzed C-N cross-coupling reactions to date. Often low catalyst loadings and short reaction times can be used with functionalized aryl and heteroaryl coupling partners. The reactions are highly robust and can be set up and performed without the use of a glovebox. These catalysts should find wide application in the synthesis of complex molecules including pharmaceuticals, natural products and functional materials.
Figures
Similar articles
-
Highly reactive, general and long-lived catalysts for palladium-catalyzed amination of heteroaryl and aryl chlorides, bromides, and iodides: scope and structure-activity relationships.J Am Chem Soc. 2008 May 21;130(20):6586-96. doi: 10.1021/ja077074w. Epub 2008 Apr 30. J Am Chem Soc. 2008. PMID: 18444639 Free PMC article.
-
A highly active catalyst for Pd-catalyzed amination reactions: cross-coupling reactions using aryl mesylates and the highly selective monoarylation of primary amines using aryl chlorides.J Am Chem Soc. 2008 Oct 15;130(41):13552-4. doi: 10.1021/ja8055358. Epub 2008 Sep 18. J Am Chem Soc. 2008. PMID: 18798626 Free PMC article.
-
Mechanism-Driven Development of Group 10 Metal-Catalyzed Decarbonylative Coupling Reactions.Acc Chem Res. 2022 Dec 6;55(23):3430-3444. doi: 10.1021/acs.accounts.2c00496. Epub 2022 Nov 16. Acc Chem Res. 2022. PMID: 36382937 Free PMC article.
-
Recent progress in coupling of two heteroarenes.Chemistry. 2011 May 9;17(20):5466-92. doi: 10.1002/chem.201003039. Epub 2011 Apr 19. Chemistry. 2011. PMID: 21506178 Review.
-
Palladium-based catalytic systems for the synthesis of conjugated enynes by sonogashira reactions and related alkynylations.Angew Chem Int Ed Engl. 2007;46(6):834-71. doi: 10.1002/anie.200602761. Angew Chem Int Ed Engl. 2007. PMID: 17335070 Review.
Cited by
-
Amination of heteroaryl chlorides: palladium catalysis or SN Ar in green solvents?ChemSusChem. 2013 Aug;6(8):1455-60. doi: 10.1002/cssc.201300239. Epub 2013 Jun 21. ChemSusChem. 2013. PMID: 23794470 Free PMC article.
-
Important Role of NH-Carbazole in Aryl Amination Reactions Catalyzed by 2-Aminobiphenyl Palladacycles.ACS Catal. 2023 Mar 7;13(6):3934-3948. doi: 10.1021/acscatal.3c00075. eCollection 2023 Mar 17. ACS Catal. 2023. PMID: 36970467 Free PMC article.
-
Palladium and Copper: Advantageous Nanocatalysts for Multi-Step Transformations.Nanomaterials (Basel). 2021 Jul 23;11(8):1891. doi: 10.3390/nano11081891. Nanomaterials (Basel). 2021. PMID: 34443727 Free PMC article. Review.
-
Application of Cyclic Diaryliodonium Salts in the Synthesis of Axially Chiral Natural Product Analogues.Org Lett. 2024 Jun 28;26(25):5258-5262. doi: 10.1021/acs.orglett.4c01308. Epub 2024 Jun 17. Org Lett. 2024. PMID: 38885455 Free PMC article.
-
Pd-Catalyzed Decarbonylative Cross-Couplings of Aroyl Chlorides.Org Lett. 2017 Aug 4;19(15):4142-4145. doi: 10.1021/acs.orglett.7b02024. Epub 2017 Jul 19. Org Lett. 2017. PMID: 28723158 Free PMC article.
References
-
- Marion N, Nolan SP. Acc. Chem. Res. 2008;41:1440–1449. - PubMed
-
- Tasler S, Mies J, Langa M. Adv. Synth. Catal. 2007;349:2286–2300.
-
- Torborg C, Beller M. Adv. Synth. Catal. 2009;351:3027–3043.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources