Identification of yeast genes involved in k homeostasis: loss of membrane traffic genes affects k uptake
- PMID: 22384317
- PMCID: PMC3276120
- DOI: 10.1534/g3.111.000166
Identification of yeast genes involved in k homeostasis: loss of membrane traffic genes affects k uptake
Abstract
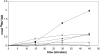
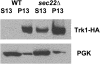
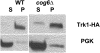
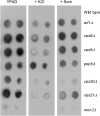
Using the homozygous diploid Saccharomyces deletion collection, we searched for strains with defects in K(+) homeostasis. We identified 156 (of 4653 total) strains unable to grow in the presence of hygromycin B, a phenotype previously shown to be indicative of ion defects. The most abundant group was that with deletions of genes known to encode membrane traffic regulators. Nearly 80% of these membrane traffic defective strains showed defects in uptake of the K(+) homolog, (86)Rb(+). Since Trk1, a plasma membrane protein localized to lipid microdomains, is the major K(+) influx transporter, we examined the subcellular localization and Triton-X 100 insolubility of Trk1 in 29 of the traffic mutants. However, few of these showed defects in the steady state levels of Trk1, the localization of Trk1 to the plasma membrane, or the localization of Trk1 to lipid microdomains, and most defects were mild compared to wild-type. Three inositol kinase mutants were also identified, and in contrast, loss of these genes negatively affected Trk1 protein levels. In summary, this work reveals a nexus between K(+) homeostasis and membrane traffic, which does not involve traffic of the major influx transporter, Trk1.
Keywords: TRK1; VPS genes.
Figures




Similar articles
-
Trk1 and Trk2 define the major K(+) transport system in fission yeast.J Bacteriol. 2000 Jan;182(2):394-9. doi: 10.1128/JB.182.2.394-399.2000. J Bacteriol. 2000. PMID: 10629185 Free PMC article.
-
Erv14 cargo receptor participates in regulation of plasma-membrane potential, intracellular pH and potassium homeostasis via its interaction with K+-specific transporters Trk1 and Tok1.Biochim Biophys Acta Mol Cell Res. 2019 Sep;1866(9):1376-1388. doi: 10.1016/j.bbamcr.2019.05.005. Epub 2019 May 25. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31136755
-
TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae.J Bacteriol. 1994 Jan;176(1):249-52. doi: 10.1128/jb.176.1.249-252.1994. J Bacteriol. 1994. PMID: 8282703 Free PMC article.
-
The Schizosaccharomyces pombe Pzh1 protein phosphatase regulates Na+ ion influx in a Trk1-independent fashion.Eur J Biochem. 1999 Feb;260(1):31-7. doi: 10.1046/j.1432-1327.1999.00129.x. Eur J Biochem. 1999. PMID: 10091581
-
A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter.Mol Cell Biol. 1999 May;19(5):3328-37. doi: 10.1128/MCB.19.5.3328. Mol Cell Biol. 1999. PMID: 10207057 Free PMC article.
Cited by
-
Cis and trans interactions between genes encoding PAF1 complex and ESCRT machinery components in yeast.Curr Genet. 2018 Oct;64(5):1105-1116. doi: 10.1007/s00294-018-0828-6. Epub 2018 Mar 22. Curr Genet. 2018. PMID: 29564528 Free PMC article.
-
Exomer Is Part of a Hub Where Polarized Secretion and Ionic Stress Connect.Front Microbiol. 2021 Jul 19;12:708354. doi: 10.3389/fmicb.2021.708354. eCollection 2021. Front Microbiol. 2021. PMID: 34349749 Free PMC article.
-
The CDK8 kinase module: A novel player in the transcription of translation initiation and ribosomal genes.Mol Biol Cell. 2025 Jan 1;36(1):ar2. doi: 10.1091/mbc.E24-04-0164. Epub 2024 Nov 20. Mol Biol Cell. 2025. PMID: 39565680 Free PMC article.
-
Small GTPase proteins in macroautophagy.Small GTPases. 2018 Sep 3;9(5):409-414. doi: 10.1080/21541248.2016.1246280. Epub 2016 Nov 1. Small GTPases. 2018. PMID: 27763811 Free PMC article. Review.
-
The roles of monomeric GTP-binding proteins in macroautophagy in Saccharomyces cerevisiae.Int J Mol Sci. 2014 Oct 9;15(10):18084-101. doi: 10.3390/ijms151018084. Int J Mol Sci. 2014. PMID: 25302616 Free PMC article. Review.
References
-
- Adams A., Gottschling D. E., Kaiser C. A., Stearns T., 1997. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Plainview, NY
-
- Ali R., Brett C. L., Mukherjee S., Rao R., 2004. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279: 4498–4506 - PubMed
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., et al. , 1997. Short Protocols in Molecular Biology. John Wiley & Sons, Inc., New York
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
