Whole-Genome Sequencing of Sordaria macrospora Mutants Identifies Developmental Genes
- PMID: 22384404
- PMCID: PMC3284333
- DOI: 10.1534/g3.111.001479
Whole-Genome Sequencing of Sordaria macrospora Mutants Identifies Developmental Genes
Abstract
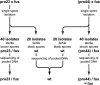
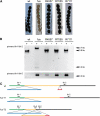
The study of mutants to elucidate gene functions has a long and successful history; however, to discover causative mutations in mutants that were generated by random mutagenesis often takes years of laboratory work and requires previously generated genetic and/or physical markers, or resources like DNA libraries for complementation. Here, we present an alternative method to identify defective genes in developmental mutants of the filamentous fungus Sordaria macrospora through Illumina/Solexa whole-genome sequencing. We sequenced pooled DNA from progeny of crosses of three mutants and the wild type and were able to pinpoint the causative mutations in the mutant strains through bioinformatics analysis. One mutant is a spore color mutant, and the mutated gene encodes a melanin biosynthesis enzyme. The causative mutation is a G to A change in the first base of an intron, leading to a splice defect. The second mutant carries an allelic mutation in the pro41 gene encoding a protein essential for sexual development. In the mutant, we detected a complex pattern of deletion/rearrangements at the pro41 locus. In the third mutant, a point mutation in the stop codon of a transcription factor-encoding gene leads to the production of immature fruiting bodies. For all mutants, transformation with a wild type-copy of the affected gene restored the wild-type phenotype. Our data demonstrate that whole-genome sequencing of mutant strains is a rapid method to identify developmental genes in an organism that can be genetically crossed and where a reference genome sequence is available, even without prior mapping information.
Keywords: Sordaria macrospora; developmental mutants; next-generation sequencing.
Figures





References
-
- Bell A. A., Wheeler M. H., 1986. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 24: 411–451
LinkOut - more resources
Full Text Sources
