Biodistribution and safety assessment of bladder cancer specific recombinant oncolytic adenovirus in subcutaneous xenografts tumor model in nude mice
- PMID: 22384806
- PMCID: PMC3684264
- DOI: 10.2174/156652312800099599
Biodistribution and safety assessment of bladder cancer specific recombinant oncolytic adenovirus in subcutaneous xenografts tumor model in nude mice
Abstract
Background: The previous works about safety evaluation for constructed bladder tissue specific adenovirus are poorly documented. Thus, we investigated the biodistribution and body toxicity of bladder specific oncolytic adenovirus Ad-PSCAE-UPII-E1A (APU-E1A) and Ad-PSCAE-UPII-E1A-AR (APU-E1A-AR), providing meaningful information prior to embarking on human clinical trials.
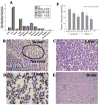
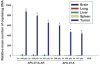
Materials and method: Conditionally replicate recombinant adenovirus (CRADs) APU-E1A, APU-EIA-AR were constructed with bladder tissue specific UroplakinII(UPII) promoter to induce the expression of Ad5E1A gene and E1A-AR fusing gene, and PSCAE was inserted at upstream of promoter to enhance the function of promoter. Based on the cytopathic and anti-tumor effect of bladder cancer, these CRADs were intratumorally injected into subcutaneous xenografts tumor in nude mice. We then determined the toxicity through general health and behavioral assessment, hepatic and hematological toxicity evaluation, macroscopic and microscopic postmortem analyses. The spread of the transgene E1A of adenovirus was detected with RT-PCR and Western blot. Virus replication and distribution were examined with APU-LUC administration and Luciferase Assay.
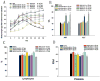
Results: General assessment and body weight of the animals did not reveal any alteration in general behavior. The hematological alterations of groups which were injected with 5x10(8) pfu or higher dose (5x10(9) pfu) of APU-E1A and APU-E1A-AR showed no difference in comparison with PBS group, and only slight increased transaminases in contrast to PBS group at 5x10(9) pfu of APU-E1A and APU-E1A-AR were observed. E1A transgene did not disseminate to organs outside of xenograft tumor. Virus replication was not detected in other organs beside tumor according to Luciferase Assay.
Conclusions: Our study showed that recombinant adenovirus APU-E1A-AR and APU-E1A appear safe with 5x10(7) pfu and 5x10(8) pfu intratumorally injection in mice, without any discernable effects on general health and behavior.
Conflict of interest statement
Figures


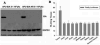
References
-
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. - PubMed
-
- Ghoneim MA, Abol-Enein H. Management of muscle-invasive bladder cancer: an update. Nat Clin Pract Urol. 2008;5:501–8. - PubMed
-
- ABCM-a C. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–34. - PubMed
-
- Hussain SA, James ND. The systemic treatment of advanced and metastatic bladder cancer. Lancet Oncol. 2003;4:489–97. - PubMed
-
- Karamouzis MV, Argiris A, Grandis JR. Clinical applications of gene therapy in head and neck cancer. Curr Gene Ther. 2007;7:446–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

