Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice
- PMID: 22384859
- PMCID: PMC3385033
- DOI: 10.1111/j.1365-2567.2012.03585.x
Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice
Abstract
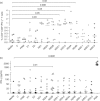
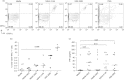
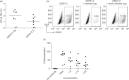
Dengue is a mosquito-borne viral disease of humans, and animal models that recapitulate human immune responses or dengue pathogenesis are needed to understand the pathogenesis of the disease. We recently described an animal model for dengue virus (DENV) infection using humanized NOD-scid IL2rγ(null) mice (NSG) engrafted with cord blood haematopoietic stem cells. We sought to further improve this model by co-transplantation of human fetal thymus and liver tissues into NSG (BLT-NSG) mice. Enhanced DENV-specific antibody titres were found in the sera of BLT-NSG mice compared with human cord blood haematopoietic stem cell-engrafted NSG mice. Furthermore, B cells generated during the acute phase and in memory from splenocytes of immunized BLT-NSG mice secreted DENV-specific IgM antibodies with neutralizing activity. Human T cells in engrafted BLT-NSG mice secreted interferon-γ in response to overlapping DENV peptide pools and HLA-A2 restricted peptides. The BLT-NSG mice will allow assessment of human immune responses to DENV vaccines and the effects of previous immunity on subsequent DENV infections.
© 2012 The Authors. Immunology © 2012 Blackwell Publishing Ltd.
Figures


Similar articles
-
Dengue virus infection induces broadly cross-reactive human IgM antibodies that recognize intact virions in humanized BLT-NSG mice.Exp Biol Med (Maywood). 2015 Jan;240(1):67-78. doi: 10.1177/1535370214546273. Epub 2014 Aug 14. Exp Biol Med (Maywood). 2015. PMID: 25125497 Free PMC article.
-
Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice.PLoS One. 2009 Oct 5;4(10):e7251. doi: 10.1371/journal.pone.0007251. PLoS One. 2009. PMID: 19802382 Free PMC article.
-
Utility of humanized BLT mice for analysis of dengue virus infection and antiviral drug testing.J Virol. 2014 Feb;88(4):2205-18. doi: 10.1128/JVI.03085-13. Epub 2013 Dec 11. J Virol. 2014. PMID: 24335303 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
Dengue Vaccines: The Promise and Pitfalls of Antibody-Mediated Protection.Cell Host Microbe. 2021 Jan 13;29(1):13-22. doi: 10.1016/j.chom.2020.12.011. Cell Host Microbe. 2021. PMID: 33444553 Review.
Cited by
-
Flying under the radar - impact and factors influencing asymptomatic DENV infections.Front Cell Infect Microbiol. 2023 Nov 24;13:1284651. doi: 10.3389/fcimb.2023.1284651. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38076464 Free PMC article. Review.
-
Dengue vaccines: recent developments, ongoing challenges and current candidates.Expert Rev Vaccines. 2013 Aug;12(8):933-53. doi: 10.1586/14760584.2013.815412. Expert Rev Vaccines. 2013. PMID: 23984962 Free PMC article. Review.
-
Translating Treg Therapy in Humanized Mice.Front Immunol. 2015 Dec 14;6:623. doi: 10.3389/fimmu.2015.00623. eCollection 2015. Front Immunol. 2015. PMID: 26697017 Free PMC article. Review.
-
Application of Humanized Mice in Immunological Research.Methods Mol Biol. 2016;1371:157-76. doi: 10.1007/978-1-4939-3139-2_10. Methods Mol Biol. 2016. PMID: 26530800 Free PMC article. Review.
-
CD141+ dendritic cells produce prominent amounts of IFN-α after dsRNA recognition and can be targeted via DEC-205 in humanized mice.Blood. 2013 Jun 20;121(25):5034-44. doi: 10.1182/blood-2012-12-473413. Epub 2013 Mar 12. Blood. 2013. PMID: 23482932 Free PMC article.
References
-
- Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of pre-existing dengue virus (DV) neutralizing antibody levels to viremia and disease severity in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. - PubMed
-
- Laoprasopwattana K, Libraty DH, Endy TP, et al. Antibody dependent cellular cytotoxicity in pre-secondary dengue virus serotype 3 (DV3) but not in DV2 infection plasma samples inversely correlated with viremia levels. J Infect Dis. 2007;195:1108–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

