Chemotherapy targeting by DNA capture in viral protein particles
- PMID: 22385197
- PMCID: PMC3429352
- DOI: 10.2217/nnm.11.104
Chemotherapy targeting by DNA capture in viral protein particles
Erratum in
- Nanomedicine (Lond). 2012 Aug;7(8):1275-6
Abstract
Aim: This study tests the hypothesis that DNA intercalation and electrophilic interactions can be exploited to noncovalently assemble doxorubicin in a viral protein nanoparticle designed to target and penetrate tumor cells through ligand-directed delivery. We further test whether this new paradigm of doxorubicin targeting shows therapeutic efficacy and safety in vitro and in vivo.
Materials & methods: We tested serum stability, tumor targeting and therapeutic efficacy in vitro and in vivo using biochemical, microscopy and cytotoxicity assays.
Results: Self-assembly formed approximately 10-nm diameter serum-stable nanoparticles that can target and ablate HER2+ tumors at >10× lower dose compared with untargeted doxorubicin, while sparing the heart after intravenous delivery. The targeted nanoparticle tested here allows doxorubicin potency to remain unaltered during assembly, transport and release into target cells,while avoiding peripheral tissue damage and enabling lower, and thus safer, drug dose for tumor killing.
Conclusion: This nanoparticle may be an improved alternative to chemical conjugates and signal-blocking antibodies for tumor-targeted treatment.
Figures


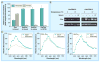



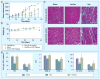

Similar articles
-
Analysis of targeted viral protein nanoparticles delivered to HER2+ tumors.J Vis Exp. 2013 Jun 18;(76):50396. doi: 10.3791/50396. J Vis Exp. 2013. PMID: 23851334 Free PMC article.
-
Nano-delivery vehicle based on chlorin E6, photodynamic therapy, doxorubicin chemotherapy provides targeted treatment of HER-2 negative, ανβ3-positive breast cancer.Pharmacol Res. 2020 Oct;160:105184. doi: 10.1016/j.phrs.2020.105184. Epub 2020 Sep 15. Pharmacol Res. 2020. PMID: 32946931
-
Impact of tumor HER2/ERBB2 expression level on HER2-targeted liposomal doxorubicin-mediated drug delivery: multiple low-affinity interactions lead to a threshold effect.Mol Cancer Ther. 2013 Sep;12(9):1816-28. doi: 10.1158/1535-7163.MCT-13-0180. Epub 2013 May 30. Mol Cancer Ther. 2013. PMID: 23723124
-
pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances.Nanomedicine (Lond). 2014 Mar;9(3):487-99. doi: 10.2217/nnm.13.212. Nanomedicine (Lond). 2014. PMID: 24746192 Review.
-
Dextran-doxorubicin/chitosan nanoparticles for solid tumor therapy.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009 Jul-Aug;1(4):415-25. doi: 10.1002/wnan.43. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009. PMID: 20049807 Review.
Cited by
-
Analysis of targeted viral protein nanoparticles delivered to HER2+ tumors.J Vis Exp. 2013 Jun 18;(76):50396. doi: 10.3791/50396. J Vis Exp. 2013. PMID: 23851334 Free PMC article.
-
HER3-targeted protein chimera forms endosomolytic capsomeres and self-assembles into stealth nucleocapsids for systemic tumor homing of RNA interference in vivo.Nucleic Acids Res. 2019 Dec 2;47(21):11020-11043. doi: 10.1093/nar/gkz900. Nucleic Acids Res. 2019. PMID: 31617560 Free PMC article.
-
Systemic HER3 ligand-mimicking nanobioparticles enter the brain and reduce intracranial tumour growth.Nat Nanotechnol. 2025 May;20(5):683-696. doi: 10.1038/s41565-025-01867-7. Epub 2025 Feb 21. Nat Nanotechnol. 2025. PMID: 39984637 Free PMC article.
-
A corrole nanobiologic elicits tissue-activated MRI contrast enhancement and tumor-targeted toxicity.J Control Release. 2015 Nov 10;217:92-101. doi: 10.1016/j.jconrel.2015.08.046. Epub 2015 Aug 31. J Control Release. 2015. PMID: 26334483 Free PMC article.
-
Cell Membrane-Based Biomimetic Nanoparticles and the Immune System: Immunomodulatory Interactions to Therapeutic Applications.Front Bioeng Biotechnol. 2020 Jun 17;8:627. doi: 10.3389/fbioe.2020.00627. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32626700 Free PMC article. Review.
References
-
- Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010;53(2):94–104. - PubMed
-
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. - PubMed
-
- Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100(1):135–144. - PubMed
-
- Yoo HS, Park TG. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J Control Release. 2004;100(2):247–256. - PubMed
-
- Glockshuber R, Malia M, Pfitzinger I, Pluckthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990;29(6):1362–1367. - PubMed
Website
-
- Demonstration of daily tail vein injections. www.youtube.com/watch?v=zbqjZ2HL_-0.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
