Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies
- PMID: 22385232
- PMCID: PMC3390488
- DOI: 10.1111/j.1365-2249.2011.04555.x
Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies
Abstract
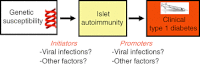
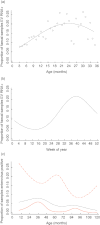
The hypothesis that under some circumstances enteroviral infections can lead to type 1 diabetes (T1D) was proposed several decades ago, based initially on evidence from animal studies and sero-epidemiology. Subsequently, enterovirus RNA has been detected more frequently in serum of patients than in control subjects, but such studies are susceptible to selection bias and reverse causality. Here, we review critically recent evidence from human studies, focusing on longitudinal studies with potential to demonstrate temporal association. Among seven longitudinal birth cohort studies, the evidence that enterovirus infections predict islet autoimmunity is quite inconsistent in our interpretation, due partially, perhaps, to heterogeneity in study design and a limited number of subjects studied. An association between enterovirus and rapid progression from autoimmunity to T1D was reported by one longitudinal study, but although consistent with evidence from animal models, this novel observation awaits replication. It is possible that a potential association with initiation and/or progression of islet autoimmunity can be ascribed to a subgroup of the many enterovirus serotypes, but this has still not been investigated properly. There is a need for larger studies with frequent sample intervals and collection of specimens of sufficient quality and quantity for detailed characterization of enterovirus. More research into the molecular epidemiology of enteroviruses and enterovirus immunity in human populations is also warranted. Ultimately, this knowledge may be used to devise strategies to reduce the risk of T1D in humans.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures

References
-
- Stene LC, Tuomilehto J, Rewers M. Global epidemiology of type 1 diabetes. In: Ekoé J-M, Rewers M, Williams R, Zimmet P, editors. The epidemiology of diabetes mellitus. Chichester: Wiley-Blackwell; 2008. pp. 355–83.
-
- Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–9. - PubMed
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. - PubMed
-
- Shibasaki S, Imagawa A, Tauriainen S, et al. Expression of toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

