Control of Mycobacterium tuberculosis growth by activated natural killer cells
- PMID: 22385249
- PMCID: PMC3390505
- DOI: 10.1111/j.1365-2249.2011.04552.x
Control of Mycobacterium tuberculosis growth by activated natural killer cells
Abstract
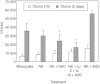
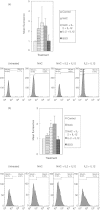
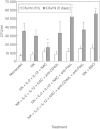
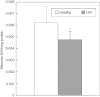
We characterized the underlying mechanisms by which glutathione (GSH)-enhanced natural killer (NK) cells inhibit the growth of Mycobacterium tuberculosis (M. tb) inside human monocytes. We observed that in healthy individuals, treatment of NK cells with N-acetyl cysteine (NAC), a GSH prodrug in conjunction with cytokines such as interleukin (IL)-2 + IL-12, resulted in enhanced expression of NK cytotoxic ligands (FasL and CD40L) with concomitant stasis in the intracellular growth of M. tb. Neutralization of FasL and CD40L in IL-2 + IL-12 + NAC-treated NK cells resulted in abrogation in the growth inhibition of M. tb inside monocytes. Importantly, we observed that the levels of GSH are decreased significantly in NK cells derived from individuals with HIV infection compared to healthy subjects, and this decrease correlated with a several-fold increase in the growth of M. tb inside monocytes. This study describes a novel innate defence mechanism adopted by NK cells to control M. tb infection.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures



References
-
- Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology. 2010;15:413–32. - PubMed
-
- Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257(4 (Pt 1)):L163–73. - PubMed
-
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–35. - PubMed
-
- Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008;300:423–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

