Tolerance to lipopolysaccharide promotes an enhanced neutrophil extracellular traps formation leading to a more efficient bacterial clearance in mice
- PMID: 22385250
- PMCID: PMC3390506
- DOI: 10.1111/j.1365-2249.2012.04560.x
Tolerance to lipopolysaccharide promotes an enhanced neutrophil extracellular traps formation leading to a more efficient bacterial clearance in mice
Abstract
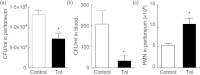
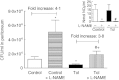
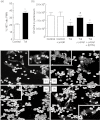
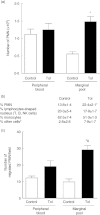
Tolerance to lipopolysaccharide (LPS) constitutes a stress adaptation, in which a primary contact with LPS results in a minimal response when a second exposure with the same stimulus occurs. However, active important defence mechanisms are mounted during the tolerant state. Our aim was to assess the contribution of polymorphonuclear neutrophils (PMN) in the clearance of bacterial infection in a mouse model of tolerance to LPS. After tolerance was developed, we investigated in vivo different mechanisms of bacterial clearance. The elimination of a locally induced polymicrobial challenge was more efficient in tolerant mice both in the presence or absence of local macrophages. This was related to a higher number of PMN migrating to the infectious site as a result of an increased number of PMN from the marginal pool with higher chemotactic capacity, not because of differences in their phagocytic activity or reactive species production. In vivo, neutrophils extracellular trap (NET) destruction by nuclease treatment abolished the observed increased clearance in tolerant but not in control mice. In line with this finding, in vitro NETs formation was higher in PMN from tolerant animals. These results indicate that the higher chemotactic response from an increased PMN marginal pool and the NETs enhanced forming capacity are the main mechanisms mediating bacterial clearance in tolerant mice. To sum up, far from being a lack of response, tolerance to LPS causes PMN priming effects which favour distant and local anti-infectious responses.
© 2012 The Authors. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures

References
-
- Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–83. - PubMed
-
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53. - PubMed
-
- Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

