Catalytic mechanism of aromatic prenylation by NphB
- PMID: 22385275
- PMCID: PMC3314166
- DOI: 10.1021/bi201800m
Catalytic mechanism of aromatic prenylation by NphB
Abstract
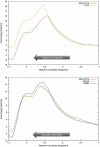
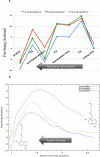
NphB is an aromatic prenyltransferase that catalyzes the attachment of a 10-carbon geranyl group to aromatic substrates. Importantly, NphB exhibits a rich substrate selectivity and product regioselectivity. A systematic computational study has been conducted in order to address several question associated with NphB-catalyzed geranylation. The reaction mechanism of the prenylation step has been characterized as a S(N)1 type dissociative mechanism with a weakly stable carbocation intermediate. A novel π-chamber composed of Tyr121, Tyr216, and 1,6-DHN is found to be important in stabilizing the carbocation. The observed difference in the rates of product formation from 5- and 2-prenylation arises from the differing orientations of the aromatic substrate in the resting state. 4-Prenylation shares the same resting state with 5-prenylation, but the lower free energy barrier for carbocation formation makes the latter reaction more facile. The high free energy barrier associated with 7-prenylation is caused by the unfavorable orientation of 1,6-DHN in active site pocket, along with the difficulty of proton elimination after the prenylation step. A water-mediated proton transfer facilitates the loss of hydrogen at the prenylation site to form the final prenylated product. Interestingly, the same crystallographically observed water molecule has been found to be responsible for proton loss in all three experimentally identified products. After proton transfer, the relaxation of the final product from a sp(3) carbon center to a sp(2) center triggers a "spring-loaded" product release mechanism which pushes the final product out of the binding pocket toward the edge of the active site. The hydrogen bond interactions between the two hydroxyl groups of the aromatic product and the side chains of Ser214 and Tyr288 help to "steer" the movement of the product. In addition, mutagenesis studies identify these same two side chains as being responsible for the observed regioselectivity, particularly 2-prenylation. These observations provide valuable insights into NphB chemistry, offering an opportunity to better engineer the active site and to control the reactivity in order to obtain high yields of the desired product(s). Furthermore, the S(N)1 reaction mechanism observed for NphB differs from the prenylation reaction found in, for example, the farnesyltransferase, which proceeds via an S(N)2-like reaction pathway. The spring-loaded release mechanism highlighted herein also offers novel insights into how enzymes facilitate product release.
Figures
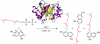
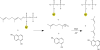

Similar articles
-
Acceptor substrate determines donor specificity of an aromatic prenyltransferase: expanding the biocatalytic potential of NphB.Appl Microbiol Biotechnol. 2020 May;104(10):4383-4395. doi: 10.1007/s00253-020-10529-8. Epub 2020 Mar 18. Appl Microbiol Biotechnol. 2020. PMID: 32189045 Free PMC article.
-
Understanding the substrate selectivity and the product regioselectivity of Orf2-catalyzed aromatic prenylations.Biochemistry. 2007 Feb 6;46(5):1303-11. doi: 10.1021/bi062076z. Biochemistry. 2007. PMID: 17260959
-
Chemoenzymatic syntheses of prenylated aromatic small molecules using Streptomyces prenyltransferases with relaxed substrate specificities.Bioorg Med Chem. 2008 Sep 1;16(17):8117-26. doi: 10.1016/j.bmc.2008.07.052. Epub 2008 Jul 24. Bioorg Med Chem. 2008. PMID: 18682327 Free PMC article.
-
Role of substrate dynamics in protein prenylation reactions.Acc Chem Res. 2015 Feb 17;48(2):439-48. doi: 10.1021/ar500321u. Epub 2014 Dec 24. Acc Chem Res. 2015. PMID: 25539152 Free PMC article. Review.
-
Catalytic mechanism and engineering of aromatic prenyltransferase: A review.Int J Biol Macromol. 2025 Jun;313:144214. doi: 10.1016/j.ijbiomac.2025.144214. Epub 2025 May 14. Int J Biol Macromol. 2025. PMID: 40379159 Review.
Cited by
-
Origin of product selectivity in a prenyl transfer reaction from the same intermediate: exploration of multiple FtmPT1-catalyzed prenyl transfer pathways.Biochemistry. 2014 Sep 30;53(38):6126-38. doi: 10.1021/bi500747z. Epub 2014 Sep 16. Biochemistry. 2014. PMID: 25188320 Free PMC article.
-
Structure-based engineering increased the catalytic turnover rate of a novel phenazine prenyltransferase.PLoS One. 2012;7(10):e48427. doi: 10.1371/journal.pone.0048427. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119011 Free PMC article.
-
Structure of a membrane-embedded prenyltransferase homologous to UBIAD1.PLoS Biol. 2014 Jul 22;12(7):e1001911. doi: 10.1371/journal.pbio.1001911. eCollection 2014 Jul. PLoS Biol. 2014. PMID: 25051182 Free PMC article.
-
Biosynthesis of novel cannabigerolic acid derivatives by engineering the substrate specificity of aromatic prenyltransferase.Front Bioeng Biotechnol. 2025 Apr 10;13:1563708. doi: 10.3389/fbioe.2025.1563708. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40276033 Free PMC article.
-
Rational Design and Modification of NphB for Cannabinoids Biosynthesis.Molecules. 2024 Sep 19;29(18):4454. doi: 10.3390/molecules29184454. Molecules. 2024. PMID: 39339449 Free PMC article.
References
-
- Zhang FL, Casey PJ. Protein Prenylation: Molecular Mechanisms and Functional Consequences. Annu. Rev. Biochem. 1996;65:241–269. - PubMed
-
- Park HW, Boduluri SR, Moomaw JF, Casey PJ, Beese LS. Crystal structure of protein farnesyltransferase at 2.25 angstrom resolution. Science. 1997;275:1800–1804. - PubMed
-
- Long SB, Casey PJ, Beese LS. Reaction path of protein farnesyltransferase at atomic resolution. Nature. 2002;419:645–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

