Proteolytic regulation of the mitochondrial cAMP-dependent protein kinase
- PMID: 22385295
- PMCID: PMC3309167
- DOI: 10.1021/bi201573k
Proteolytic regulation of the mitochondrial cAMP-dependent protein kinase
Abstract
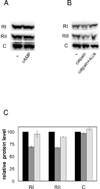
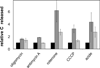
The mitochondrial cAMP-dependent protein kinase (PKA) is activatable in a cAMP-independent fashion. The regulatory (R) subunits of the PKA holoenzyme (R(2)C(2)), but not the catalytic (C) subunits, suffer proteolysis upon exposure of bovine heart mitochondria to digitonin, Ca(2+), and a myriad of electron transport inhibitors. Selective loss of both the RI- and RII-type subunits was demonstrated via Western blot analysis, and activation of the C subunit was revealed by phosphorylation of a validated PKA peptide substrate. Selective proteolysis transpires in a calpain-dependent fashion as demonstrated by exposure of the R and C subunits of PKA to calpain and by attenuation of R and C subunit proteolysis in the presence of calpain inhibitor I. By contrast, exposure of mitochondria to cAMP fails to promote R subunit degradation, although it does result in enhanced C subunit catalytic activity. Treatment of mitochondria with electron transport chain inhibitors rotenone, antimycin A, sodium azide, and oligomycin, as well as an uncoupler of oxidative phosphorylation, also elicits enhanced C subunit activity. These results are consistent with the notion that signals, originating from cAMP-independent sources, elicit enhanced mitochondrial PKA activity.
Figures





References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Gullingsrud J, Kim C, Taylor SS, McCammon JA. Dynamic binding of PKA regulatory subunit RI alpha. Structure. 2006;14:141–149. - PubMed
-
- Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–696. - PubMed
-
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. - PubMed
-
- Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T. Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J Biol Chem. 2001;276:20827–20830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

