Nickel-catalyzed cross-coupling of chromene acetals and boronic acids
- PMID: 22385385
- PMCID: PMC4804347
- DOI: 10.1021/ol300364s
Nickel-catalyzed cross-coupling of chromene acetals and boronic acids
Abstract
A modular and highly efficient protocol for the synthesis of 2-aryl- and heteroaryl-2H-chromenes is described. Under base-free conditions, readily accessible 2-ethoxy-2H-chromenes undergo C(sp(3))-O activation and C(sp(3))-C bond formation in the presence of an inexpensive nickel catalyst and boronic acids. This new strategy enables broad access to 2-substituted-2H-chromenes and has been applied to the late-stage incorporation of complex molecules, including the pharmaceuticals loratidine and indomethacin methyl ester.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


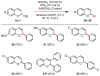

Feasibility independent of electronic/steric properties of reaction partners
First example of Suzuki–Miyaura cross-coupling with allylic acetals

References
-
- Lago JHG, Ramos CS, Casanova DCC, Morandim AD, Bergamo DCB, Cavalheiro AJ, Bolzani VD, Furlan M, Guimaraes EF, Young MCM, Kato MJ. J Nat Prod. 2004;67:1783–1788. - PubMed
-
- Mukai K, Okabe K, Hosose H. J Org Chem. 1989;54:557–560.
-
- Gauthier S, Caron B, Cloutier J, Dory YL, Favre A, Larouche D, Mailhot J, Ouellet C, Schwerdtfeger A, Leblanc G, Martel C, Simard J, Merand Y, Belanger A, Labrie C, Labrie F. J Med Chem. 1997;40:2117–2122. - PubMed
-
- Schneider P, Hawser S, Islam K. Biorg Med Chem Lett. 2003;13:4217–4221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

