Understanding substrate selectivity of human UDP-glucuronosyltransferases through QSAR modeling and analysis of homologous enzymes
- PMID: 22385482
- PMCID: PMC4390583
- DOI: 10.3109/00498254.2012.663515
Understanding substrate selectivity of human UDP-glucuronosyltransferases through QSAR modeling and analysis of homologous enzymes
Abstract
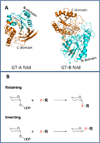
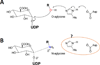
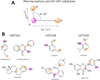
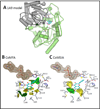
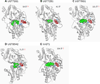
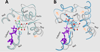
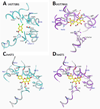
The UDP-glucuronosyltransferase (UGT) enzyme catalyzes the glucuronidation reaction which is a major metabolic and detoxification pathway in humans. Understanding the mechanisms for substrate recognition by UGT assumes great importance in an attempt to predict its contribution to xenobiotic/drug disposition in vivo. Spurred on by this interest, 2D/3D-quantitative structure activity relationships and pharmacophore models have been established in the absence of a complete mammalian UGT crystal structure. This review discusses the recent progress in modeling human UGT substrates including those with multiple sites of glucuronidation. A better understanding of UGT active site contributing to substrate selectivity (and regioselectivity) from the homologous enzymes (i.e. plant and bacterial UGTs, all belong to family 1 of glycosyltransferase (GT1)) is also highlighted, as these enzymes share a common catalytic mechanism and/or overlapping substrate selectivity.
Figures


References
-
- Battaglia E, Senay C, Fournel-Gigleux S, Herber R, Siest G, Magdalou J. The chemical modification of human liver UDP-glucuronosyltransferase UGT1*6 reveals the involvement of a carboxyl group in catalysis. FEBS Lett. 1994;346(2–3):146–150. - PubMed
-
- Bolam DN, Roberts S, Proctor MR, Turkenburg JP, Dodson EJ, Martinez-Fleites C, Yang M, Davis BG, Davies GJ, Gilbert HJ. The crystal structure of two macrolide glycosyltransferases provides a blueprint for host cell antibiotic immunity. Proc Natl Acad Sci U S A. 2007;104(13):5336–5341. - PMC - PubMed
-
- Bosma PJ. Inherited disorders of bilirubin metabolism. J Hepatol. 2003;38:107–117. - PubMed
-
- Strassburg CP. Pharmacogenetics of Gilbert’s syndrome. Pharmacogenomics. 2008;9:703–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
