DNA damage response pathways and cell cycle checkpoints in colorectal cancer: current concepts and future perspectives for targeted treatment
- PMID: 22385513
- PMCID: PMC7458274
- DOI: 10.2174/156800912800190901
DNA damage response pathways and cell cycle checkpoints in colorectal cancer: current concepts and future perspectives for targeted treatment
Abstract
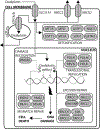
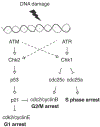
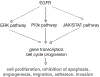
Although several drugs have been designed in the last few years to target specific key pathways and functions in colorectal cancer (CRC), the backbone of CRC treatment is still made up of compounds which rely on DNA damage to accomplish their role. DNA damage response (DDR) and checkpoint pathways are intertwined signaling networks that arrest cell cycle, recognize and repair genetic mistakes which arise during DNA replication and transcription, as well as through the exposure to chemical and physical agents that interact with nucleic acids. The good but highly variable activity of DNA damaging agents in the treatment of CRC suggests that intrinsic alterations in DDR pathways and cell cycle checkpoints may contribute differentially to the way cancer cells react to DNA damage. In the present review, our aim is to depict the recent advances in understanding the molecular basis of the activity of DNA damaging agents used for the treatment of CRC. We focus on the known and potential drug targets that are part of these complex and intertwined pathways. We describe the potential role of the checkpoints in CRC, and how their pharmacological manipulation could lead to chemopotentiation or synergism with currently used drugs. Novel therapeutic agents playing a role in DDR and checkpoint inhibition are assessed. We discuss the possible rationale for combining PARP inhibition with DNA damaging agents, and we address the link between DDR and EGFR pathways in CRC.
Conflict of interest statement
CONFLICT OF INTEREST
None.
Figures
Similar articles
-
DNA damage response and prostate cancer: defects, regulation and therapeutic implications.Oncogene. 2015 May 28;34(22):2815-22. doi: 10.1038/onc.2014.238. Epub 2014 Aug 18. Oncogene. 2015. PMID: 25132269 Free PMC article. Review.
-
Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor.Carcinogenesis. 2013 Nov;34(11):2486-97. doi: 10.1093/carcin/bgt240. Epub 2013 Jul 3. Carcinogenesis. 2013. PMID: 23825154
-
Poly(ADP-ribose) polymerase inhibition enhances p53-dependent and -independent DNA damage responses induced by DNA damaging agent.Cell Cycle. 2011 Dec 1;10(23):4074-82. doi: 10.4161/cc.10.23.18170. Epub 2011 Dec 1. Cell Cycle. 2011. PMID: 22101337 Free PMC article.
-
The synthetic lethality of targeting cell cycle checkpoints and PARPs in cancer treatment.J Hematol Oncol. 2022 Oct 17;15(1):147. doi: 10.1186/s13045-022-01360-x. J Hematol Oncol. 2022. PMID: 36253861 Free PMC article. Review.
-
Jumonji domain-containing protein 2B silencing induces DNA damage response via STAT3 pathway in colorectal cancer.Br J Cancer. 2014 Feb 18;110(4):1014-26. doi: 10.1038/bjc.2013.808. Epub 2014 Jan 28. Br J Cancer. 2014. PMID: 24473398 Free PMC article.
Cited by
-
CT-based radiogenomic analysis dissects intratumor heterogeneity and predicts prognosis of colorectal cancer: a multi-institutional retrospective study.J Transl Med. 2022 Dec 8;20(1):574. doi: 10.1186/s12967-022-03788-8. J Transl Med. 2022. PMID: 36482390 Free PMC article.
-
Small molecule 2,3-DCPE induces S phase arrest by activating the ATM/ATR-Chk1-Cdc25A signaling pathway in DLD-1 colon cancer cells.Oncol Lett. 2020 Dec;20(6):294. doi: 10.3892/ol.2020.12157. Epub 2020 Sep 25. Oncol Lett. 2020. PMID: 33101488 Free PMC article.
-
USP25 Elevates SHLD2-Mediated DNA Double-Strand Break Repair and Regulates Chemoresponse in Cancer.Adv Sci (Weinh). 2024 Jul;11(28):e2403485. doi: 10.1002/advs.202403485. Epub 2024 May 27. Adv Sci (Weinh). 2024. PMID: 38803048 Free PMC article.
-
On biomarkers and pathways in rectal cancer: What's the target?World J Gastrointest Surg. 2012 Dec 27;4(12):275-7. doi: 10.4240/wjgs.v4.i12.275. World J Gastrointest Surg. 2012. PMID: 23493582 Free PMC article.
-
Identification and Characterization of MortaparibPlus-A Novel Triazole Derivative That Targets Mortalin-p53 Interaction and Inhibits Cancer-Cell Proliferation by Wild-Type p53-Dependent and -Independent Mechanisms.Cancers (Basel). 2021 Feb 17;13(4):835. doi: 10.3390/cancers13040835. Cancers (Basel). 2021. PMID: 33671256 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

