Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells
- PMID: 22385573
- PMCID: PMC3392769
- DOI: 10.1186/scrt100
Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells
Abstract
Introduction: The capacity of bone marrow mesenchymal stromal cells (BMSCs) to be induced into chondrocytes has drawn much attention for cell-based cartilage repair. BMSCs represent a small proportion of cells of the bone marrow stromal compartment and, thus, culture expansion is a necessity for therapeutic use. However, there is no consensus on how BMSCs should be isolated nor expanded to maximize their chondrogenic potential. During embryonic development pluripotent stem cells differentiate into chondrocytes and form cartilage in a hypoxic microenvironment.
Methods: Freshly harvested human BMSCs were isolated and expanded from the aspirates of six donors, under either hypoxic conditions (3% O2) or normoxic conditions (21% O2). A colony-forming unit fibroblastic (Cfu-f) assay was used to determine the number of cell colonies developed from each donor. BMSCs at passage 2 (P2) were characterized by flow cytometry for the phenotypic expression of cell surface markers on mesenchymal stem cells. BMSCs at P2 were subsequently cultured in vitro as three-dimensional cell pellets in a defined serum-free chondrogenic medium under normoxic and hypoxic conditions. Chondrogenic differentiation of the BMSCs was characterized by biochemical and histological methods and by quantitative gene-expression analysis.
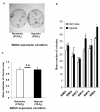
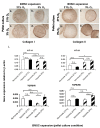
Results: After 14 days of culture, the number of BMSC colonies developed under hypoxia was generally higher (8% to 38% depending on donor) than under normoxia. BMSCs were positive for the cell surface markers CD13, CD29, CD44, CD73, CD90, CD105 and CD151, and negative for CD34. Regardless of the oxygen tension during pellet culture, hypoxia-expanded BMSC pellets underwent a more robust chondrogenesis than normoxia-expanded BMSC pellets after three weeks of culture, as judged by increased glycosaminoglycan synthesis and Safranin O staining, along with increased mRNA expression of aggrecan, collagen II and Sox9. Hypoxic conditions enhanced the mRNA expression of hypoxia inducible factor-2 alpha (HIF-2α) but suppressed the mRNA expression of collagen X in BMSC pellet cultures regardless of the oxygen tension during BMSC isolation and propagation.
Conclusions: Taken together, our data demonstrate that isolation and expansion of BMSCs under hypoxic conditions augments the chondrogenic potential of BMSCs. This suggests that hypoxia-mediated isolation and expansion of BMSCs may improve clinical applications of BMSCs for cartilage repair.
Figures




References
-
- Watt FM. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci. 1988;89:373–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

