Comparison of Gd-Bz-TTDA, Gd-EOB-DTPA, and Gd-BOPTA for dynamic MR imaging of the liver in rat models
- PMID: 22385605
- PMCID: PMC11915929
- DOI: 10.1016/j.kjms.2011.10.017
Comparison of Gd-Bz-TTDA, Gd-EOB-DTPA, and Gd-BOPTA for dynamic MR imaging of the liver in rat models
Abstract
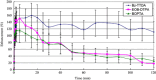
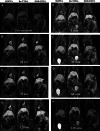
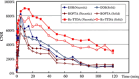
To evaluate the competitive potential of a new lipophilic paramagnetic complex, Gd-Bz-TTDA [4-benzyl-3,6,10-tri (carboxymethyl)-3,6,10-triazado-decanedioic acid] compared with two other commercially available MR hepatobiliary contrast agents, gadobenate dimeglumine (Gd-BOPTA) and gadoxetic acid (Gd-EOB-DTPA), dynamic MR imaging studies were performed on normal and hepatocellular carcinoma (HCC) rat models using a 1.5-Tesla MR scanner. The results indicate that normal rats that were injected with 0.1 mmol/kg Gd-Bz-TTDA showed significantly more intense and persistent liver enhancement than those that were injected with the same dose of Gd-EOB-DTPA or Gd-BOPTA. All of these agents showed similar enhancement patterns in the implanted HCC. The liver-lesion contrast-to-noise ratios were higher and more persistent in rats that were injected with Gd-Bz-TTDA. These results indicate that Gd-Bz-TTDA is comparable with the commercially available hepatobiliary agents, Gd-EOB-DTPA and Gd-BOPTA, and can result in more intense and prolonged liver enhancement while still providing better liver-lesion discrimination. These results warrant further large-scale studies.
Copyright © 2012. Published by Elsevier B.V.
Figures

Similar articles
-
Evaluation of [Gd(Bz-TTDA)]2- as a potential contrast agent in MR imaging of the hepatobiliary system: an animal study.J Magn Reson Imaging. 2004 Oct;20(4):632-9. doi: 10.1002/jmri.20151. J Magn Reson Imaging. 2004. PMID: 15390149
-
Comparison of the Ability of Gadobenate Dimeglumine and Gadolinium Ethoxybenzyl Dimeglumine to Display the major Features for Noninvasively Diagnosing Hepatocellular Carcinoma According to the LI-RADS 2018v.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241260331. doi: 10.1177/15330338241260331. Technol Cancer Res Treat. 2024. PMID: 38860337 Free PMC article.
-
Hepatic hemangiomas: difference in enhancement pattern on 3T MR imaging with gadobenate dimeglumine versus gadoxetate disodium.Eur J Radiol. 2012 Oct;81(10):2457-62. doi: 10.1016/j.ejrad.2011.10.014. Epub 2011 Dec 3. Eur J Radiol. 2012. PMID: 22138122
-
Liver. III: Gadolinium-based hepatobiliary contrast agents (Gd-EOB-DTPA and Gd-BOPTA/Dimeg).Magn Reson Imaging Clin N Am. 1996 Feb;4(1):61-72. Magn Reson Imaging Clin N Am. 1996. PMID: 8673717 Review.
-
[Current status of MRI diagnostics with liver-specific contrast agents. Gd-EOB-DTPA and Gd-BOPTA].Radiologe. 2004 Dec;44(12):1185-91. doi: 10.1007/s00117-004-1134-5. Radiologe. 2004. PMID: 15549226 Review. German.
Cited by
-
Predicting Therapeutic Efficacy of Vascular Disrupting Agent CA4P in Rats with Liver Tumors by Hepatobiliary Contrast Agent Mn-DPDP-Enhanced MRI.Transl Oncol. 2020 Jan;13(1):92-101. doi: 10.1016/j.tranon.2019.09.010. Epub 2019 Dec 3. Transl Oncol. 2020. PMID: 31810003 Free PMC article.
-
Radiologic Evaluation and Structured Reporting Form for Extrahepatic Bile Duct Cancer: 2019 Consensus Recommendations from the Korean Society of Abdominal Radiology.Korean J Radiol. 2021 Jan;22(1):41-62. doi: 10.3348/kjr.2019.0803. Epub 2020 Aug 28. Korean J Radiol. 2021. PMID: 32901457 Free PMC article. Review.
-
Dynamic Contrast-Enhanced Magnetic Resonance Imaging with Gd-EOB-DTPA for the Evaluation of Liver Fibrosis Induced by Carbon Tetrachloride in Rats.PLoS One. 2015 Jun 15;10(6):e0129621. doi: 10.1371/journal.pone.0129621. eCollection 2015. PLoS One. 2015. PMID: 26076199 Free PMC article.
References
-
- Seale M.K., Catalano O.A., Saini S., Hahn P.F., Vahani D.V.. Hepatobiliary‐specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009; 29: 1725–1748. - PubMed
-
- Semlka R.C., Helmberger T.K.. Contrast agents for MR imaging of the liver. Radiology. 2001; 218: 27–38. - PubMed
-
- Kühn J.P., Hegenscheid K., Seigmund W., Froehlich C.P., Hosten N., Puls R.. Normal dynamic MRI enhancement patterns of the upper abdominal organs: gadoxetic acid compared with gadobutrol. AJR Am J Roentgenol. 2009; 193: 1318–1323. - PubMed
-
- Ba‐Ssalamah A., Uffmann M., Saini S., Bastati N., Herold C., Schima W.. Clinical value of MRI liver‐specific contrast agents: a tailored examination for a confident non‐invasive diagnosis of focal liver lesions. Eur Radiol. 2009; 19: 342–357. - PubMed
-
- Ahn S.S., Kim M.J., Js Lim, Hong H.S., Chung Y.E., Choi J.Y.. Added value of gadoxetic acid‐enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010; 255: 459–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

