Functional antagonism between Sall4 and Plzf defines germline progenitors
- PMID: 22385656
- PMCID: PMC3299297
- DOI: 10.1016/j.stem.2012.02.004
Functional antagonism between Sall4 and Plzf defines germline progenitors
Abstract
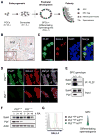
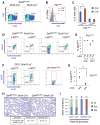
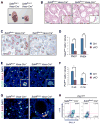
Transcription factors required for formation of embryonic tissues often maintain their expression in adult stem cell populations, but whether their function remains equivalent is not clear. Here we demonstrate critical and distinct roles for Sall4 in development of embryonic germ cells and differentiation of postnatal spermatogonial progenitor cells (SPCs). In differentiating SPCs, Sall4 levels transiently increase and Sall4 physically interacts with Plzf, a transcription factor exclusively required for adult stem cell maintenance. Mechanistically, Sall4 sequesters Plzf to noncognate chromatin domains to induce expression of Kit, a target of Plzf-mediated repression required for differentiation. Plzf in turn antagonizes Sall4 function by displacing Sall4 from cognate chromatin to induce Sall1 expression. Taken together, these data suggest that transcription factors required for embryonic tissue development postnatally take on distinct roles through interaction with opposing factors, which hence define properties of the adult stem cell compartment.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25:166–172. - PubMed
-
- Barna M, Merghoub T, Costoya JA, Ruggero D, Branford M, Bergia A, Samori B, Pandolfi PP. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510. - PubMed
-
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. - PubMed
-
- Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 Is a Novel Diagnostic Marker for Testicular Germ Cell Tumors. Am J Surg Pathol 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

