Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis
- PMID: 22385657
- PMCID: PMC3338251
- DOI: 10.1016/j.stem.2012.01.016
Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis
Abstract
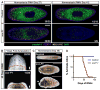
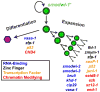
Pluripotency is a central, well-studied feature of embryonic development, but the role of pluripotent cell regulation in somatic tissue regeneration remains poorly understood. In planarians, regeneration of entire animals from tissue fragments is promoted by the activity of adult pluripotent stem cells (cNeoblasts). We utilized transcriptional profiling to identify planarian genes expressed in adult proliferating, regenerative cells (neoblasts). We also developed quantitative clonal analysis methods for expansion and differentiation of cNeoblast descendants that, together with RNAi, revealed gene roles in stem cell biology. Genes encoding two zinc finger proteins, Vasa, a LIM domain protein, Sox and Jun-like transcription factors, two candidate RNA-binding proteins, a Setd8-like protein, and PRC2 (Polycomb) were required for proliferative expansion and/or differentiation of cNeoblast-derived clones. These findings suggest that planarian stem cells utilize molecular mechanisms found in germ cells and other pluripotent cell types and identify genetic regulators of the planarian stem cell system.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Comment in
-
The planarian Prometheus, quantified.Nat Methods. 2012 May;9(5):432-3. doi: 10.1038/nmeth.2005. Nat Methods. 2012. PMID: 22803200 No abstract available.
References
-
- Bardeen C, Baetjer F. The inhibitive action of the Roentgen rays on regeneration in planarians. J Exp Zool. 1904;1:191–195.
-
- Boyer L, Plath K, Zeitlinger J, Brambrink T, Medeiros L, Lee T, Levine S, Wernig M, Tajonar A, Ray M, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

