No systemic reactions to influenza vaccination in egg-sensitized tertiary-care pediatric patients
- PMID: 22385977
- PMCID: PMC3313878
- DOI: 10.1186/1710-1492-8-2
No systemic reactions to influenza vaccination in egg-sensitized tertiary-care pediatric patients
Abstract
Background: There are numerous, disparate guidelines for influenza vaccination in egg-allergic patients. We aimed to describe the outcome of selectively applied guidelines, based on risk-stratification, to our high risk, egg-allergic, tertiary-care pediatric population.
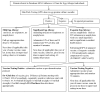
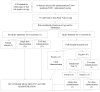
Methods: Egg allergy was confirmed with skin testing. The vaccine administered was an adjuvunated 2009 H1N1 influenza A vaccine with < 0.165 mcg/ml ovalbumin. Patients with mild egg allergy were to receive the vaccination in 1 dose, those with severe egg allergy were to receive 2 split doses, and patients with exquisite egg allergy or significant co-morbidities were to be skin tested with the vaccine (prick full strength, intradermal 1:100 of final concentration without adjuvant) and had 5 step desensitization if the testing was positive, or 1-2 step administration if negative. Patients were observed for 60 minutes after the final dose and anaphylaxis treatment was available. We report the frequency of allergic reactions.
Results: Ninety-nine patients were referred and 79 had positive egg testing. Asthma was present in 67% and 30% had prior anaphylaxis to egg. We vaccinated 77 of 79 patients: 71 without performing vaccine skin testing. Two refused vaccination. No patient had a systemic reaction or required treatment. Two patients experienced positive testing to the adjuvanated intradermal vaccine, but were negative without adjuvant.
Conclusions: Our results suggest that most egg-allergic tertiary care pediatric patients can be vaccinated with a low ovalbumin content influenza vaccine without prior vaccine testing. Vaccine skin testing, if used at all, can be reserved for special circumstances. The squalene adjuvant may cause an irritant reaction with intradermal testing.
Figures
Similar articles
-
Assessment of epicutaneous testing of a monovalent Influenza A (H1N1) 2009 vaccine in egg allergic patients.Allergy Asthma Clin Immunol. 2011 Feb 11;7(1):3. doi: 10.1186/1710-1492-7-3. Allergy Asthma Clin Immunol. 2011. PMID: 21314925 Free PMC article.
-
Safe vaccination of patients with egg allergy with an adjuvanted pandemic H1N1 vaccine.J Allergy Clin Immunol. 2010 Aug;126(2):317-23. doi: 10.1016/j.jaci.2010.05.037. Epub 2010 Jun 25. J Allergy Clin Immunol. 2010. PMID: 20579720 Clinical Trial.
-
Safe administration of influenza vaccine to patients with egg allergy.J Pediatr. 1998 Nov;133(5):624-8. doi: 10.1016/s0022-3476(98)70101-5. J Pediatr. 1998. PMID: 9821418 Clinical Trial.
-
Establishing the safety of influenza vaccine in egg-allergic individuals.Pediatr Ann. 2013 Jul;42(7):122-7. doi: 10.3928/00904481-20130619-09. Pediatr Ann. 2013. PMID: 23805959 Review.
-
An update on influenza vaccination in patients with egg allergy.Curr Opin Pediatr. 2011 Oct;23(5):566-72. doi: 10.1097/MOP.0b013e32834ac7a3. Curr Opin Pediatr. 2011. PMID: 21881505 Review.
Cited by
-
Allergic Reactions to Vaccines in Children: From Constituents to Specific Vaccines.Biomedicines. 2023 Feb 18;11(2):620. doi: 10.3390/biomedicines11020620. Biomedicines. 2023. PMID: 36831156 Free PMC article. Review.
-
Vaccines and allergic reactions: The past, the current COVID-19 pandemic, and future perspectives.Allergy. 2021 Jun;76(6):1640-1660. doi: 10.1111/all.14840. Epub 2021 Jun 4. Allergy. 2021. PMID: 33811364 Free PMC article. Review.
-
CoronaVac/Sinovac COVID-19 Vaccine-Related Hypersensitivity Reactions and Second-Dose Vaccine Administration: Tertiary Allergy Center Experience.Int Arch Allergy Immunol. 2022;183(7):778-784. doi: 10.1159/000524099. Epub 2022 Apr 22. Int Arch Allergy Immunol. 2022. PMID: 35462361 Free PMC article.
-
Successful Desensitization to mRNA COVID-19 Vaccine in a Case Series of Patients With a History of Anaphylaxis to the First Vaccine Dose.Front Allergy. 2022 Feb 2;3:825164. doi: 10.3389/falgy.2022.825164. eCollection 2022. Front Allergy. 2022. PMID: 35386647 Free PMC article.
-
Vaccination in children with allergy to non active vaccine components.Clin Transl Med. 2015 Feb 14;4:3. doi: 10.1186/s40169-014-0043-0. eCollection 2015. Clin Transl Med. 2015. PMID: 25852819 Free PMC article.
References
-
- Pickering LK, Baker CJ, Kimberlin DW, Long SS, editor. American Academy of Pediatrics; Influenza. Red book: 2009 report of the Committee on Infectious Diseases. 28. Elk Grove Village (IL): American Academy of Pediatrics; 2009. pp. 400–412.
-
- Canadian Immunization Guide 2006. 7. Ottawa: Public Health Agency of Canada; 2006. Influenza Vaccine; pp. 209–20.http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-inf-eng.php
-
- Anolik RA, Spiegel W, Posner M, Jakabovics E. Influenza vaccine testing in egg sensitive patients [letter] Ann Allergy. 1992;68:9. - PubMed
LinkOut - more resources
Full Text Sources