Remote noninvasive allograft rejection monitoring for heart transplant recipients: study protocol for the novel evaluation with home electrocardiogram and remote transmission (NEW HEART) study
- PMID: 22386040
- PMCID: PMC3323456
- DOI: 10.1186/1471-2261-12-14
Remote noninvasive allograft rejection monitoring for heart transplant recipients: study protocol for the novel evaluation with home electrocardiogram and remote transmission (NEW HEART) study
Abstract
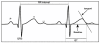
Background: Acute allograft rejection is a major cause of early mortality in the first year after heart transplantation in adults. Although endomyocardial biopsy (EMB) is not a perfect "gold standard" for a correct diagnosis of acute allograft rejection, it is considered the best available test and thus, is the current standard practice. Unfortunately, EMB is an invasive and costly procedure that is not without risk. Recent evidence suggests that acute allograft rejection causes delays in ventricular repolarization and thereby increases the cellular action potential duration resulting in a longer QT interval on the electrocardiogram (ECG). No prospective study to date has investigated whether such increases in the QT interval could provide early detection of acute allograft rejection. Therefore, in the Novel Evaluation With Home Electrocardiogram And Remote Transmission (NEW HEART) study, we plan to investigate the potential benefit of daily home QT interval monitoring to predict acute allograft rejection.
Methods/design: The NEW HEART study is a prospective, double-blind, multi-center descriptive research study. A sample of 325 adult heart transplant recipients will be recruited within six weeks of transplant from three sites in the United States. Subjects will receive the HeartView™ ECG recorder and its companion Internet Transmitter, which will transmit the subject's ECG to a Core Laboratory. Subjects will be instructed to record and transmit an ECG recording daily for 6 months. An increase in the QTC interval from the previous day of at least 25 ms that persists for 3 consecutive days will be considered abnormal. The number and grade of acute allograft rejection episodes, as well as all-cause mortality, will be collected for one year following transplant surgery.
Discussion: This study will provide "real world" prospective data to determine the sensitivity and specificity of QTC as an early non invasive marker of cellular rejection in transplant recipients during the first post-transplant year. A non-invasive indicator of early allograft rejection in heart transplant recipients has the potential to limit the number and severity of rejection episodes by reducing the time and cost of rejection surveillance and by shortening the time to recognition of rejection.
Trial registration: ClinicalTrials.gov: NCT01365806.
Figures
Similar articles
-
Usefulness of the QTc interval in predicting acute allograft rejection.Thorac Cardiovasc Surg. 1998 Aug;46(4):217-21. doi: 10.1055/s-2007-1010228. Thorac Cardiovasc Surg. 1998. PMID: 9776496
-
Feasibility and compliance with daily home electrocardiogram monitoring of the QT interval in heart transplant recipients.Heart Lung. 2012 Jul-Aug;41(4):368-73. doi: 10.1016/j.hrtlng.2012.02.012. Epub 2012 Mar 28. Heart Lung. 2012. PMID: 22459508 Free PMC article.
-
[Predictive value of ECG changes for acute cardiac rejections in heart transplant recipients].Med Klin (Munich). 2006 Feb 15;101(2):99-106. doi: 10.1007/s00063-006-1014-z. Med Klin (Munich). 2006. PMID: 16501906 German.
-
European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation.Eur Heart J Cardiovasc Imaging. 2015 Sep;16(9):919-48. doi: 10.1093/ehjci/jev139. Epub 2015 Jul 2. Eur Heart J Cardiovasc Imaging. 2015. PMID: 26139361 Review.
-
The challenge of endomyocardial biopsy interpretation in assessing cardiac allograft rejection.Curr Opin Cardiol. 1997 Mar;12(2):146-52. doi: 10.1097/00001573-199703000-00009. Curr Opin Cardiol. 1997. PMID: 9192483 Review.
Cited by
-
Perceived control and health-related quality of life in heart transplant recipients.Eur J Cardiovasc Nurs. 2018 Aug;17(6):513-520. doi: 10.1177/1474515117749225. Epub 2017 Dec 20. Eur J Cardiovasc Nurs. 2018. PMID: 29260888 Free PMC article.
-
Electrocardiographic abnormalities in the first year after heart transplantation.J Electrocardiol. 2014 Mar-Apr;47(2):135-9. doi: 10.1016/j.jelectrocard.2013.09.006. Epub 2013 Oct 10. J Electrocardiol. 2014. PMID: 24119878 Free PMC article. Clinical Trial.
-
Clinical and gender differences in heart transplant recipients in the NEW HEART study.Eur J Cardiovasc Nurs. 2017 Mar;16(3):222-229. doi: 10.1177/1474515116651178. Epub 2016 Jul 8. Eur J Cardiovasc Nurs. 2017. PMID: 27189203 Free PMC article.
-
[Heart and combined heart-lung transplantation. Indications, chances and risks].Herz. 2014 Feb;39(1):66-73. doi: 10.1007/s00059-013-4042-5. Herz. 2014. PMID: 24452762 German.
-
Non-invasive detection of cardiac allograft rejection among heart transplant recipients using an electrocardiogram based deep learning model.Eur Heart J Digit Health. 2023 Jan 13;4(2):71-80. doi: 10.1093/ehjdh/ztad001. eCollection 2023 Mar. Eur Heart J Digit Health. 2023. PMID: 36974261 Free PMC article.
References
-
- The Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov/data/
-
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth adult heart transplant report - 2011. The Journal of Heart and Lung Transplantation. 2011;30(10):1078–1094. doi: 10.1016/j.healun.2011.08.003. - DOI - PubMed
-
- Fiorelli AI, Coelho GHB, Oliveira JL Jr, Aiello VD, Benvenuti LA, Santos A, Chi A, Tallans A, Igushi ML, Bacal F. et al.Endomyocardial Biopsy as Risk Factor in the Development of Tricuspid Insufficiency After Heart Transplantation. Transplant Proc. 2009;41(3):935–937. doi: 10.1016/j.transproceed.2009.02.011. - DOI - PubMed