Association between inhaled nitric oxide treatment and long-term pulmonary function in survivors of acute respiratory distress syndrome
- PMID: 22386043
- PMCID: PMC3681348
- DOI: 10.1186/cc11215
Association between inhaled nitric oxide treatment and long-term pulmonary function in survivors of acute respiratory distress syndrome
Abstract
Introduction: Assessment of treatments for acute respiratory distress syndrome (ARDS) has focused on short-term outcomes (for example, mortality); little information exists regarding long-term effects of ARDS treatment. Survivors of ARDS episodes may have long-term obstructive/restrictive pulmonary abnormalities and pulmonary gas exchange impairment. A 2004 prospective randomized placebo-controlled trial assessed the efficacy and safety of inhaled nitric oxide (iNO) in patients with non-septic ARDS; the primary endpoint was days alive and off assisted breathing. This analysis examined potential effects of iNO or placebo on pulmonary function six months post-treatment in ARDS survivors from that original study.
Methods: ARDS survivors (N = 92) from a large-scale randomized, placebo-controlled study evaluating mortality after either 5 ppm iNO or placebo for up to 28 days were assessed six months post-treatment. Pulmonary function testing across seven parameters was conducted.
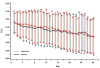
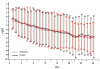
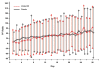
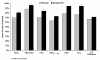
Results: At 6 months post-treatment, results indicated significantly better absolute values for iNO versus placebo for mean ± SD total lung capacity (TLC, 5.54 ± 1.42 vs. 4.81 ± 1.00; P = 0.026). There were also significantly better values for mean ± SD percent predicted values for a) forced expiratory volume in 1 second (FEV1, 80.23 ± 21.21 vs. 69.51 ± 28.97; P = 0.042), b) forced vital capacity (FVC, 83.78 ± 19.37 vs. 69.84 ± 27.40; P = 0.019), c) FEV1/FVC (96.14 ± 13.79 vs. 87.92 ± 19.77; P = 0.033), and d) TLC (93.33 ± 18.21 vs. 76.10 ± 21.84; P < 0.001). Nonsignificant differences were found in absolute FEV1, FEV1/FVC, FVC, forced expiratory flow from 25% to 75% of FVC, functional residual capacity, and CO diffusion.
Conclusions: ARDS patients surviving after treatment with low-dose iNO had significantly better values for select pulmonary function tests at six months post-treatment than placebo-treated patients. Further trials are warranted to determine the effects of iNO on chronic lung function in ARDS survivors, a factor in long-term morbidity and quality of life in this population.
Trial registration: A Double-blind, Randomized, Placebo-controlled, Dose-response Study of Inhaled Nitric Oxide in the Treatment of Acute Respiratory Distress Syndrome. NCT number: ISRCTN53268296.
Figures
Similar articles
-
Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group.Crit Care Med. 1998 Jan;26(1):15-23. doi: 10.1097/00003246-199801000-00011. Crit Care Med. 1998. PMID: 9428538 Clinical Trial.
-
Pulmonary function in adult survivors of severe acute lung injury treated with inhaled nitric oxide.Acta Anaesthesiol Scand. 1998 Apr;42(4):391-8. doi: 10.1111/j.1399-6576.1998.tb05131.x. Acta Anaesthesiol Scand. 1998. PMID: 9563856
-
Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial.JAMA. 2004 Apr 7;291(13):1603-9. doi: 10.1001/jama.291.13.1603. JAMA. 2004. PMID: 15069048 Clinical Trial.
-
Inhaled Nitric Oxide in Acute Respiratory Distress Syndrome Subsets: Rationale and Clinical Applications.J Aerosol Med Pulm Drug Deliv. 2023 Jun;36(3):112-126. doi: 10.1089/jamp.2022.0058. Epub 2023 Apr 20. J Aerosol Med Pulm Drug Deliv. 2023. PMID: 37083488 Free PMC article. Review.
-
Inhaled nitric oxide in ARDS.Crit Care Clin. 2002 Jan;18(1):45-68, vi. doi: 10.1016/s0749-0704(03)00064-2. Crit Care Clin. 2002. PMID: 11910732 Review.
Cited by
-
Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics.Antioxid Redox Signal. 2013 May 10;18(14):1797-809. doi: 10.1089/ars.2012.4833. Epub 2012 Oct 15. Antioxid Redox Signal. 2013. PMID: 22871207 Free PMC article. Review.
-
COVID-19: The Immune Responses and Clinical Therapy Candidates.Int J Mol Sci. 2020 Aug 3;21(15):5559. doi: 10.3390/ijms21155559. Int J Mol Sci. 2020. PMID: 32756480 Free PMC article. Review.
-
Electrochemical Generation of Nitric Oxide for Medical Applications.Electrochem Sci Adv. 2022 Oct;2(5):e2100156. doi: 10.1002/elsa.202100156. Epub 2021 Oct 25. Electrochem Sci Adv. 2022. PMID: 36386004 Free PMC article.
-
Feedback-controlled photolytic gas phase nitric oxide delivery from S-nitrosothiol-doped silicone rubber films.J Control Release. 2020 Feb;318:264-269. doi: 10.1016/j.jconrel.2019.11.030. Epub 2019 Nov 25. J Control Release. 2020. PMID: 31778741 Free PMC article.
-
Effects of inhaled nitric oxide for postoperative hypoxemia in acute type A aortic dissection: a retrospective observational study.J Cardiothorac Surg. 2020 Jan 22;15(1):25. doi: 10.1186/s13019-020-1069-6. J Cardiothorac Surg. 2020. PMID: 31969173 Free PMC article.
References
-
- INOmax [package insert] Clinton, NJ: INO Therapeutics; 2009.
-
- Blanch L, Joseph D, Fernandez R, Mas A, Martinez M, Vallés J, Diaz E, Baigorri F, Artigas A. Hemodynamic and gas exchange responses to inhalation of nitric oxide in patients with the acute respiratory distress syndrome and in hypoxemic patients with chronic obstructive pulmonary disease. Intensive Care Med. 1997;16:51–57. doi: 10.1007/s001340050290. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

