Asthmatic airway smooth muscle CXCL10 production: mitogen-activated protein kinase JNK involvement
- PMID: 22387292
- PMCID: PMC3362261
- DOI: 10.1152/ajplung.00232.2011
Asthmatic airway smooth muscle CXCL10 production: mitogen-activated protein kinase JNK involvement
Abstract
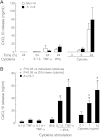
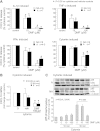
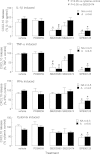
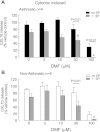
CXCL10 (IP10) is involved in mast cell migration to airway smooth muscle (ASM) bundles in asthma. We aimed to investigate the role of cytokine-induced MAPK activation in CXCL10 production by ASM cells from people with and without asthma. Confluent growth-arrested ASM cells were treated with inhibitors of the MAPKs ERK, p38, and JNK and transcription factor NF-κB, or vehicle, and stimulated with IL-1β, TNF-α, or IFN-γ, alone or combined (cytomix). CXCL10 mRNA and protein, JNK, NF-κB p65 phosphorylation, and Iκ-Bα protein degradation were assessed using real-time PCR, ELISA, and immunoblotting, respectively. Cytomix, IL-1β, and TNF-α induced CXCL10 mRNA expression more rapidly in asthmatic than nonasthmatic ASM cells. IL-1β and/or TNF-α combined with IFN-γ synergistically increased asthmatic ASM cell CXCL10 release. Inhibitor effects were similar in asthmatic and nonasthmatic cells, but cytomix-induced release was least affected, with only JNK and NF-κB inhibitors halving it. Notably, JNK phosphorylation was markedly less in asthmatic compared with nonasthmatic cells. However, in both, the JNK inhibitor SP600125 reduced JNK phosphorylation and CXCL10 mRNA levels but did not affect CXCL10 mRNA stability or Iκ-Bα degradation. Together, the JNK and NF-κB inhibitors completely inhibited their CXCL10 release. We concluded that, in asthmatic compared with nonasthmatic ASM cells, JNK activation was reduced and CXCL10 gene expression was more rapid following cytomix stimulation. However, in both, JNK activation did not regulate early events leading to NF-κB activation. Thus JNK and NF-κB provide independent therapeutic targets for limiting CXCL10 production and mast cell migration to the ASM in asthma.
Figures




References
-
- Amin K, Janson C, Boman G, Venge P. The extracellular deposition of mast cell products is increased in hypertrophic airway smooth muscles in allergic asthma but not in non-allergic asthma. Allergy 60: 1241–1247, 2005 - PubMed
-
- Baraket M, Oliver BG, Burgess JK, Dibas S, Black JL, Lim S. Relationship between alveolar macrophage derived GM-CSF, IL-10, airway hyperresponsiveness and exhaled nitric oxide in asthmatic volunteers. Proc Am Thorac Soc 3: A485, 2006
-
- Bochner B, Hudson S, Xiao H, Liu MC. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol 112: 930–934, 2003 - PubMed
-
- Brightling CE, Ammit A, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The chemokine CXCL10 (IP-10) mediates mast cell migration into the asthmatic airway smooth muscle bundle. Am J Respir Crit Care Med 171: 1103–1108, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

