Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study
- PMID: 22387567
- PMCID: PMC3408938
- DOI: 10.1093/ndt/gfs018
Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study
Abstract
Background: Optimal treatment for secondary hyperparathyroidism (SHPT) has not been defined. The IMPACT SHPT (ClinicalTrials.gov identifier: NCT00977080) study assessed whether dose-titrated paricalcitol plus supplemental cinacalcet only for hypercalcaemia is superior to cinacalcet plus low-dose vitamin D in controlling intact parathyroid hormone (iPTH) levels in patients with SHPT on haemodialysis.
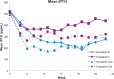
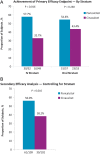
Methods: In this 28-week, multicentre, open-label Phase 4 study, participants were randomly selected to receive paricalcitol or cinacalcet plus low-dose vitamin D. Randomization and analyses were stratified by mode of paricalcitol administration [intravenous (IV) or oral]. The primary efficacy end point was the proportion of subjects who achieved a mean iPTH value of 150-300 pg/mL during Weeks 21-28.
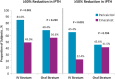
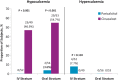
Results: Of 272 subjects randomized, 268 received one or more dose of study drug; 101 in the IV and 110 in the oral stratum with two or more values during Weeks 21-28 were included in the primary analysis. In the IV stratum, 57.7% of subjects in the paricalcitol versus 32.7% in the cinacalcet group (P = 0.016) achieved the primary end point. In the oral stratum, the corresponding proportions of subjects were 54.4% for paricalcitol and 43.4% for cinacalcet (P = 0.260). Cochran-Mantel-Haenszel analysis, controlling for stratum, revealed overall superiority of paricalcitol (56.0%) over cinacalcet (38.2%; P = 0.010) in achieving iPTH 150-300 pg/mL during Weeks 21-28. Hypercalcaemia occurred in 4 (7.7%) and 0 (0%) of paricalcitol-treated subjects in the IV and oral strata, respectively. Hypocalcaemia occurred in 46.9% and 54.7% of cinacalcet-treated subjects in the IV and oral strata, respectively.
Conclusion: Paricalcitol versus cinacalcet plus low-dose vitamin D provided superior control of iPTH, with low incidence of hypercalcaemia.
Figures
References
-
- Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2005;288:F253–F264. - PubMed
-
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875–885. - PubMed
-
- Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. - PubMed
-
- Naves-Díaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26:1938–1947. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

