Development of pluripotent stem cells for vascular therapy
- PMID: 22387745
- PMCID: PMC3595194
- DOI: 10.1016/j.vph.2012.02.010
Development of pluripotent stem cells for vascular therapy
Abstract
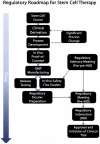
Peripheral arterial disease (PAD) is characterized by reduced limb blood flow due to arterial obstruction. Current treatment includes surgical or endovascular procedures, the failure of which may result in amputation of the affected limb. An emerging therapeutic approach is cell therapy to enhance angiogenesis and tissue survival. Small clinical trials of adult progenitor cell therapies have generated promising results, although large randomized clinical trials using well-defined cells have not been performed. Intriguing pre-clinical studies have been performed using vascular cells derived from human embryonic stem cells (hESC) or human induced pluripotent stem cells (hiPSCs). In particular, hiPSC-derived vascular cells may be a superior approach for vascular regeneration. The regulatory roadmap to the clinic will be arduous, but achievable with further understanding of the reprogramming and differentiation processes; with meticulous attention to quality control; and perseverance.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Induced pluripotent stem cells: how they will change the practice of cardiovascular medicine.Methodist Debakey Cardiovasc J. 2013 Oct-Dec;9(4):206-9. doi: 10.14797/mdcj-9-4-206. Methodist Debakey Cardiovasc J. 2013. PMID: 24298311 Free PMC article. Review.
-
Reprogrammed cells for disease modeling and regenerative medicine.Annu Rev Med. 2013;64:277-90. doi: 10.1146/annurev-med-050311-163324. Annu Rev Med. 2013. PMID: 23327523 Free PMC article. Review.
-
Regenerative Medicine/Cardiac Cell Therapy: Pluripotent Stem Cells.Thorac Cardiovasc Surg. 2018 Jan;66(1):53-62. doi: 10.1055/s-0037-1608761. Epub 2017 Dec 7. Thorac Cardiovasc Surg. 2018. PMID: 29216651 Review.
-
Clinical application of vascular regenerative therapy for peripheral artery disease.Biomed Res Int. 2013;2013:179730. doi: 10.1155/2013/179730. Epub 2013 Nov 24. Biomed Res Int. 2013. PMID: 24350247 Free PMC article. Review.
-
Embryonic template-based generation and purification of pluripotent stem cell-derived cardiomyocytes for heart repair.J Cardiovasc Transl Res. 2012 Oct;5(5):566-80. doi: 10.1007/s12265-012-9391-6. Epub 2012 Jul 18. J Cardiovasc Transl Res. 2012. PMID: 22806916 Review.
Cited by
-
Targeted delivery of human iPS-ECs overexpressing IL-8 receptors inhibits neointimal and inflammatory responses to vascular injury in the rat.Am J Physiol Heart Circ Physiol. 2016 Mar 15;310(6):H705-15. doi: 10.1152/ajpheart.00587.2015. Epub 2016 Jan 22. Am J Physiol Heart Circ Physiol. 2016. PMID: 26801304 Free PMC article.
-
A compendium on peripheral arterial disease.Circ Res. 2015 Apr 24;116(9):1505-8. doi: 10.1161/CIRCRESAHA.115.306403. Circ Res. 2015. PMID: 25908724 Free PMC article. No abstract available.
-
Intermittent claudication: new targets for drug development.Drugs. 2013 Jul;73(10):999-1014. doi: 10.1007/s40265-013-0078-3. Drugs. 2013. PMID: 23775528 Review.
-
Autologous bone marrow-derived mononuclear cell therapy in Chinese patients with critical limb ischemia due to thromboangiitis obliterans: 10-year results.Stem Cell Res Ther. 2018 Feb 22;9(1):43. doi: 10.1186/s13287-018-0784-6. Stem Cell Res Ther. 2018. PMID: 29471870 Free PMC article.
-
Novel Cell-Based and Tissue Engineering Approaches for Induction of Angiogenesis as an Alternative Therapy for Diabetic Retinopathy.Int J Mol Sci. 2020 May 15;21(10):3496. doi: 10.3390/ijms21103496. Int J Mol Sci. 2020. PMID: 32429094 Free PMC article. Review.
References
-
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008;26:1276–1284. - PubMed
-
- Aicher A, Rentsch M, Sasaki K-I, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ. Res. 2007;100:581–589. - PubMed
-
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 2004;287:C572–C579. - PubMed
-
- Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335–344. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

