Poly(ADP-ribose) polymerase-1 inhibition by arsenite promotes the survival of cells with unrepaired DNA lesions induced by UV exposure
- PMID: 22387748
- PMCID: PMC3327874
- DOI: 10.1093/toxsci/kfs099
Poly(ADP-ribose) polymerase-1 inhibition by arsenite promotes the survival of cells with unrepaired DNA lesions induced by UV exposure
Abstract
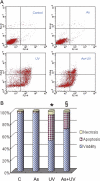
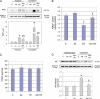
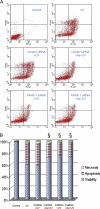
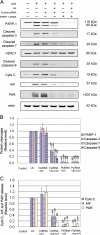
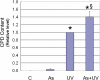
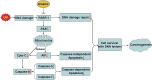
Human arsenic exposure is associated with increased risk of skin cancer, and arsenite greatly enhances ultraviolet (UV)-induced skin tumors in a mouse model of carcinogenesis. Inhibition of DNA repair is one proposed mechanism for the observed cocarcinogenicity. We have previously demonstrated that low concentrations of arsenite inhibit poly(ADP-ribose) polymerase (PARP)-1, thus interfering with DNA repair process triggered by UV radiation. Because overactivation of PARP-1 often leads to apoptotic cell death, and unrepaired DNA lesions promote genomic instability and carcinogenesis, we hypothesized that inhibition of PARP-1 by arsenic may promote the survival of potentially "initiated carcinogenic cells," i.e., cells with unrepaired DNA lesions. In the present study, we tested this hypothesis on UV-challenged HaCat cells. Cells were pretreated with 2μM arsenite for 24 h before UV exposure. Outcome parameters included apoptotic death rate, PARP-1 activation, apoptotic molecules, and retention of DNA lesions. UV exposure induced PARP-1 activation and associated poly(ADP-ribose) production, apoptosis-inducing factor release, cytochrome C release, and caspases activation, which led to apoptotic death in HaCat cells. Pretreatment with 2μM arsenite significantly inhibited UV-induced cell death as well as the associated molecular events. Notably, knockdown of PARP-1 with small interfering RNA completely abolished the antagonism of arsenite. Furthermore, arsenite pretreatment led to long-term retention of UV-induced cyclobutane pyrimidine dimers. Together, these results suggest that low concentration of arsenite reduces UV-induced apoptosis via inhibiting PARP-1, thus promoting the survival of cells with unrepaired DNA lesions, which may be an important mechanism underlying arsenic cocarcinogenic action.
Figures

References
-
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2007. - PubMed
-
- Beyersmann D, Hartwig A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008;82:493–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

