Structural insights into initial and intermediate steps of the ribosome-recycling process
- PMID: 22388519
- PMCID: PMC3321202
- DOI: 10.1038/emboj.2012.22
Structural insights into initial and intermediate steps of the ribosome-recycling process
Abstract
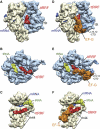
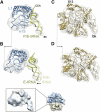
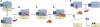
The ribosome-recycling factor (RRF) and elongation factor-G (EF-G) disassemble the 70S post-termination complex (PoTC) into mRNA, tRNA, and two ribosomal subunits. We have determined cryo-electron microscopic structures of the PoTC·RRF complex, with and without EF-G. We find that domain II of RRF initially interacts with universally conserved residues of the 23S rRNA helices 43 and 95, and protein L11 within the 50S ribosomal subunit. Upon EF-G binding, both RRF and tRNA are driven towards the tRNA-exit (E) site, with a large rotational movement of domain II of RRF towards the 30S ribosomal subunit. During this intermediate step of the recycling process, domain II of RRF and domain IV of EF-G adopt hitherto unknown conformations. Furthermore, binding of EF-G to the PoTC·RRF complex reverts the ribosome from ratcheted to unratcheted state. These results suggest that (i) the ribosomal intersubunit reorganizations upon RRF binding and subsequent EF-G binding could be instrumental in destabilizing the PoTC and (ii) the modes of action of EF-G during tRNA translocation and ribosome-recycling steps are markedly different.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
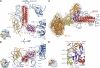
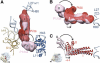

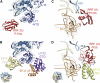
Similar articles
-
Progression of the ribosome recycling factor through the ribosome dissociates the two ribosomal subunits.Mol Cell. 2007 Jul 20;27(2):250-261. doi: 10.1016/j.molcel.2007.06.005. Mol Cell. 2007. PMID: 17643374
-
New insights into the enzymatic role of EF-G in ribosome recycling.Nucleic Acids Res. 2015 Dec 2;43(21):10525-33. doi: 10.1093/nar/gkv995. Epub 2015 Oct 1. Nucleic Acids Res. 2015. PMID: 26432831 Free PMC article.
-
Visualization of ribosome-recycling factor on the Escherichia coli 70S ribosome: functional implications.Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8900-5. doi: 10.1073/pnas.0401904101. Epub 2004 Jun 3. Proc Natl Acad Sci U S A. 2004. PMID: 15178758 Free PMC article.
-
[Ribosome recycling revisited].Mol Biol (Mosk). 2006 Jul-Aug;40(4):742-50. Mol Biol (Mosk). 2006. PMID: 16913233 Review. Russian.
-
Three-dimensional electron cryomicroscopy of ribosomes.Curr Protein Pept Sci. 2002 Feb;3(1):79-91. doi: 10.2174/1389203023380873. Curr Protein Pept Sci. 2002. PMID: 12370013 Review.
Cited by
-
Ribosome recycling defects modify the balance between the synthesis and assembly of specific subunits of the oxidative phosphorylation complexes in yeast mitochondria.Nucleic Acids Res. 2016 Jul 8;44(12):5785-97. doi: 10.1093/nar/gkw490. Epub 2016 Jun 1. Nucleic Acids Res. 2016. PMID: 27257059 Free PMC article.
-
Complete kinetic mechanism for recycling of the bacterial ribosome.RNA. 2016 Jan;22(1):10-21. doi: 10.1261/rna.053157.115. Epub 2015 Nov 2. RNA. 2016. PMID: 26527791 Free PMC article.
-
Elongation factor G is a critical target during oxidative damage to the translation system of Escherichia coli.J Biol Chem. 2012 Aug 17;287(34):28697-704. doi: 10.1074/jbc.M112.378067. Epub 2012 Jul 6. J Biol Chem. 2012. PMID: 22773838 Free PMC article.
-
Web-based visualisation and analysis of 3D electron-microscopy data from EMDB and PDB.J Struct Biol. 2013 Nov;184(2):173-81. doi: 10.1016/j.jsb.2013.09.021. Epub 2013 Oct 7. J Struct Biol. 2013. PMID: 24113529 Free PMC article.
-
Structural basis for protein synthesis: snapshots of the ribosome in motion.Curr Opin Struct Biol. 2012 Dec;22(6):743-9. doi: 10.1016/j.sbi.2012.07.011. Epub 2012 Aug 4. Curr Opin Struct Biol. 2012. PMID: 22871550 Free PMC article. Review.
References
-
- Agrawal RK, Heagle AB, Penczek P, Grassucci RA, Frank J (1999a) EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. Nat Struct Biol 6: 643–647 - PubMed
-
- Agrawal RK, Penczek P, Grassucci RA, Burkhardt N, Nierhaus KH, Frank J (1999b) Effect of buffer conditions on the position of tRNA on the 70S ribosome as visualized by cryoelectron microscopy. J Biol Chem 274: 8723–8729 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

