Structure and function of a serine carboxypeptidase adapted for degradation of the protein synthesis antibiotic microcin C7
- PMID: 22388748
- PMCID: PMC3311384
- DOI: 10.1073/pnas.1114224109
Structure and function of a serine carboxypeptidase adapted for degradation of the protein synthesis antibiotic microcin C7
Abstract
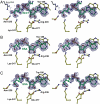
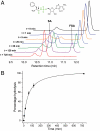
Several classes of naturally occurring antimicrobials exert their antibiotic activity by specifically targeting aminoacyl-tRNA synthetases, validating these enzymes as drug targets. The aspartyl tRNA synthetase "Trojan horse" inhibitor microcin C7 (McC7) consists of a nonhydrolyzable aspartyl-adenylate conjugated to a hexapeptide carrier that facilitates active import into bacterial cells through an oligopeptide transport system. Subsequent proteolytic processing releases the toxic compound inside the cell. Producing strains of McC7 must protect themselves against autotoxicity that may result from premature processing. The mccF gene confers resistance against endogenous and exogenous McC7 by hydrolyzing the amide bond that connects the peptide and nucleotide moieties of McC7. We present here crystal structures of MccF, in complex with various ligands. The MccF structure is similar to that of dipeptide ld-carboxypeptidase, but with an additional loop proximal to the active site that serves as the primary determinant for recognition of adenylated substrates. Wild-type MccF only hydrolyzes the naturally occurring aspartyl phosphoramidate McC7 and synthetic peptidyl sulfamoyl adenylates that contain anionic side chains. We show that substitutions of two active site MccF residues result in a specificity switch toward aromatic aminoacyl-adenylate substrates. These results suggest how MccF-like enzymes may be used to avert various toxic aminoacyl-adenylates that accumulate during antibiotic biosynthesis or in normal metabolism of the cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

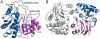

References
-
- Ibba M, Soll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. - PubMed
-
- Ling J, Reynolds N, Ibba M. Aminoacyl-tRNA synthesis and translational quality control. Annu Rev Microbiol. 2009;63:61–78. - PubMed
-
- Tao J, Schimmel P. Inhibitors of aminoacyl-tRNA synthetases as novel anti-infectives. Expert Opin Invest Drugs. 2000;9:1767–1775. - PubMed
-
- Ataide SF, Ibba M. Small molecules: Big players in the evolution of protein synthesis. ACS Chem Biol. 2006;1:285–297. - PubMed
-
- Reader JS, et al. Major biocontrol of plant tumors targets tRNA synthetase. Science. 2005;309:1533. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

