Rescue of severely affected dystrophin/utrophin-deficient mice through scAAV-U7snRNA-mediated exon skipping
- PMID: 22388933
- PMCID: PMC3349427
- DOI: 10.1093/hmg/dds082
Rescue of severely affected dystrophin/utrophin-deficient mice through scAAV-U7snRNA-mediated exon skipping
Abstract
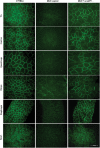
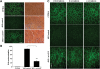
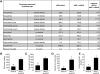
Duchenne muscular dystrophy (DMD) is a severe neuromuscular disorder caused by mutations in the dystrophin gene that result in the absence of functional protein. Antisense-mediated exon skipping is one of the most promising approaches for the treatment of DMD and recent clinical trials have demonstrated encouraging results. However, antisense oligonucleotide-mediated exon skipping for DMD still faces major hurdles such as extremely low efficacy in the cardiac muscle, poor cellular uptake and relatively rapid clearance from circulation, which means that repeated administrations are required to achieve some therapeutic efficacy. To overcome these limitations, we previously proposed the use of small nuclear RNAs (snRNAs), especially U7snRNA to shuttle the antisense sequences after vectorization into adeno-associated virus (AAV) vectors. In this study, we report for the first time the efficiency of the AAV-mediated exon skipping approach in the utrophin/dystrophin double-knockout (dKO) mouse which is a very severe and progressive mouse model of DMD. Following a single intravenous injection of scAAV9-U7ex23 in dKO mice, near-normal levels of dystrophin expression were restored in all muscles examined, including the heart. This resulted in a considerable improvement of their muscle function and dystrophic pathology as well as a remarkable extension of the dKO mice lifespan. These findings suggest great potential for AAV-U7 in systemic treatment of the DMD phenotype.
Figures
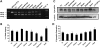
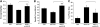

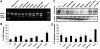
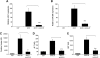
References
-
- Monaco A.P., Bertelson C.J., Liechti-Gallati S., Moser H., Kunkel L.M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. - PubMed
-
- Aartsma-Rus A., Janson A.A., Heemskerk J.A., De Winter C.L., Van Ommen G.J., Van Deutekom J.C. Therapeutic modulation of DMD splicing by blocking exonic splicing enhancer sites with antisense oligonucleotides. Ann. N. Y. Acad. Sci. 2006;1082:74–76. - PubMed
-
- van Deutekom J.C., Janson A.A., Ginjaar I.B., Frankhuizen W.S., Aartsma-Rus A., Bremmer-Bout M., den Dunnen J.T., Koop K., van der Kooi A.J., Goemans N.M., et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 2007;357:2677–2686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

