The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke
- PMID: 22388960
- PMCID: PMC3314139
- DOI: 10.1038/nn.3059
The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke
Abstract
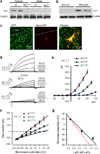
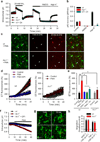
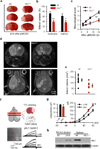
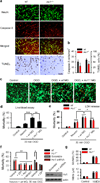
Phagocytic cell NADPH oxidase (NOX) generates reactive oxygen species (ROS) as part of innate immunity. Unfortunately, ischemia can also induce this pathway and inflict damage on native cells. The voltage-gated proton channel Hv1 enables NOX function by compensating cellular loss of electrons with protons. Accordingly, we investigated whether NOX-mediated brain damage in stroke can be inhibited by suppression of Hv1. We found that mouse and human brain microglia, but not neurons or astrocytes, expressed large Hv1-mediated currents. Hv1 was required for NOX-dependent ROS generation in brain microglia in situ and in vivo. Mice lacking Hv1 were protected from NOX-mediated neuronal death and brain damage 24 h after stroke. These results indicate that Hv1-dependent ROS production is responsible for a substantial fraction of brain damage at early time points after ischemic stroke and provide a rationale for Hv1 as a therapeutic target for the treatment of ischemic stroke.
Figures


Similar articles
-
Deficiency in the voltage-gated proton channel Hv1 increases M2 polarization of microglia and attenuates brain damage from photothrombotic ischemic stroke.J Neurochem. 2016 Oct;139(1):96-105. doi: 10.1111/jnc.13751. Epub 2016 Sep 9. J Neurochem. 2016. PMID: 27470181 Free PMC article.
-
Microglial voltage-gated proton channel Hv1 in ischemic stroke.Transl Stroke Res. 2014 Feb;5(1):99-108. doi: 10.1007/s12975-013-0289-7. Epub 2013 Oct 3. Transl Stroke Res. 2014. PMID: 24323712 Review.
-
Unconventional role of voltage-gated proton channels (VSOP/Hv1) in regulation of microglial ROS production.J Neurochem. 2017 Sep;142(5):686-699. doi: 10.1111/jnc.14106. Epub 2017 Jul 20. J Neurochem. 2017. PMID: 28628214
-
Voltage-gated proton channel HV1 in microglia.Neuroscientist. 2014 Dec;20(6):599-609. doi: 10.1177/1073858413519864. Epub 2014 Jan 23. Neuroscientist. 2014. PMID: 24463247 Review.
-
Microglial Hv1 proton channel promotes cuprizone-induced demyelination through oxidative damage.J Neurochem. 2015 Oct;135(2):347-56. doi: 10.1111/jnc.13242. Epub 2015 Aug 11. J Neurochem. 2015. PMID: 26173779 Free PMC article.
Cited by
-
A new method for evaluating regional cerebral blood flow changes: Laser speckle contrast imaging in a C57BL/6J mouse model of photothrombotic ischemia.J Huazhong Univ Sci Technolog Med Sci. 2016 Apr;36(2):174-180. doi: 10.1007/s11596-016-1562-2. Epub 2016 Apr 13. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 27072958
-
Microglia: key elements in neural development, plasticity, and pathology.J Neuroimmune Pharmacol. 2013 Jun;8(3):494-509. doi: 10.1007/s11481-013-9434-z. Epub 2013 Jan 27. J Neuroimmune Pharmacol. 2013. PMID: 23354784 Free PMC article. Review.
-
Mechanically-primed voltage-gated proton channels from angiosperm plants.Nat Commun. 2023 Nov 18;14(1):7515. doi: 10.1038/s41467-023-43280-5. Nat Commun. 2023. PMID: 37980353 Free PMC article.
-
On the role of water density fluctuations in the inhibition of a proton channel.Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):E8359-E8368. doi: 10.1073/pnas.1609964114. Epub 2016 Dec 12. Proc Natl Acad Sci U S A. 2016. PMID: 27956641 Free PMC article.
-
Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1.J Gen Physiol. 2015 Nov;146(5):343-56. doi: 10.1085/jgp.201511456. Epub 2015 Oct 12. J Gen Physiol. 2015. PMID: 26458876 Free PMC article.
References
-
- Nathan C, Ding A. SnapShot: Reactive Oxygen Intermediates (ROI) Cell. 2010;140:951–951. e952. - PubMed
-
- Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. - PubMed
-
- Walder CE, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

