Subcellular dynamics of multifunctional protein regulation: mechanisms of GAPDH intracellular translocation
- PMID: 22388977
- PMCID: PMC3350569
- DOI: 10.1002/jcb.24113
Subcellular dynamics of multifunctional protein regulation: mechanisms of GAPDH intracellular translocation
Abstract
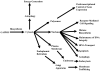
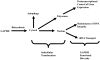
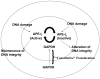
Multidimensional proteins such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) exhibit distinct activities unrelated to their originally identified functions. Apart from glycolysis, GAPDH participates in iron metabolism, membrane trafficking, histone biosynthesis, the maintenance of DNA integrity and receptor mediated cell signaling. Further, multifunctional proteins exhibit distinct changes in their subcellular localization reflecting their new activities. As such, GAPDH is not only a cytosolic protein but is localized in the membrane, the nucleus, polysomes, the ER and the Golgi. In addition, although the initial subcellular localizations of multifunctional proteins may be of significance, dynamic changes in intracellular distribution may occur as a consequence of those new activities. As such, regulatory mechanisms may exist through which cells control multifunctional protein expression as a function of their subcellular localization. The temporal sequence through which subcellular translocation and the acquisition of new GAPDH functions is considered as well as post-translational modification as a basis for its intracellular transport.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
References
-
- Bonafe N, Gilmore-Hebert M, Folk NL, Azodi M, Zhou Y, Chambers SK. Glyceraldehyde-3-phosphate dehydrogenase binds to the AU-rich 3′untranslated region of colony-stimulating factor-1 (CSF-1) messenger RNA in human ovarian cancer cells: possible role of CSF-1 posttranscriptional regulation and tumor phenotype. Cancer Res. 2005;65:3762–3771. - PubMed
-
- Brown VM, Krynetski EJ, Krynetskaia NF, Grieger D, Mukatira ST, Murti KG, Slaughter CA, Park HW, Evans WE. A novel CRM1-mediated nuclear export signal governs nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase following genotoxic stress. J Biol Chem. 2004;279:5984–5992. - PubMed
-
- Bruns GAP, Gerald PS. Human glyceraldehyde-3-phosphate dehydrogenase in man-rodent somatic cell hybrids. Science. 1976;192:54–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials