Endothelial cell responses to micropillar substrates of varying dimensions and stiffness
- PMID: 22389314
- PMCID: PMC3324647
- DOI: 10.1002/jbm.a.34059
Endothelial cell responses to micropillar substrates of varying dimensions and stiffness
Abstract
In the vascular niche, the extracellular matrix (ECM) provides a structural scaffold with a rich ligand landscape of essential matrix proteins that supports the organization and stabilization of endothelial cells (ECs) into functional blood vessels. Many of the physical interactions between ECs and macromolecular components of the ECM occur at both the micron and submicron scale. In addition, the elasticity of the ECM has been shown to be a critical factor in the progress of the angiogenic cascade. Here, we sought to determine the effect of substrate topography and elasticity (stiffness) on EC behavior. Utilizing a unique SiO(2) substrate with an array of micropillars, we first demonstrate that micropillars with heights >3 μm significantly decrease EC adhesion and spreading. Fibronectin (Fn) patterning of 1 μm high micropillars enabled EC adhesion onto the micropillars and promoted alignment in a single-cell chain manner. We then developed a robust method to generate a soft micropillar substrate array made of polydimethylsiloxane (PDMS), similar to the SiO(2) substrate. Finally, we examined the kinetics of EC adhesion and spreading on the soft PDMS substrates compared to the stiff SiO(2) substrates. Culturing cells on the PDMS substrates demonstrated an enhanced EC elongation and alignment when compared to stiff SiO(2) with similar topographical features. We conclude that the elongation and alignment of ECs is coregulated by substrate topography and stiffness and can be harnessed to guide vascular organization.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
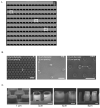
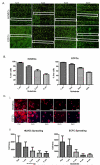

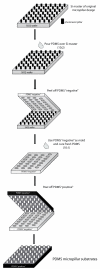

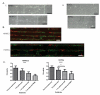
Similar articles
-
Engineering high-density endothelial cell monolayers on soft substrates.Acta Biomater. 2009 Jul;5(6):2013-24. doi: 10.1016/j.actbio.2009.01.032. Epub 2009 Feb 3. Acta Biomater. 2009. PMID: 19269269
-
Bone marrow CD34+ cell subset under induction of moderate stiffness of extracellular matrix after myocardial infarction facilitated endothelial lineage commitment in vitro.Stem Cell Res Ther. 2017 Dec 13;8(1):280. doi: 10.1186/s13287-017-0732-x. Stem Cell Res Ther. 2017. PMID: 29237495 Free PMC article.
-
The effect of substrate bulk stiffness on focal and fibrillar adhesion formation in human abdominal aortic endothelial cells.Mater Sci Eng C Mater Biol Appl. 2019 May;98:572-583. doi: 10.1016/j.msec.2018.12.130. Epub 2018 Dec 29. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30813060
-
Chemical and physical modifications to poly(dimethylsiloxane) surfaces affect adhesion of Caco-2 cells.J Biomed Mater Res A. 2010 Jun 15;93(4):1260-71. doi: 10.1002/jbm.a.32621. J Biomed Mater Res A. 2010. PMID: 19827104
-
The influence of microenvironment stiffness on endothelial cell fate: Implication for occurrence and progression of atherosclerosis.Life Sci. 2023 Dec 1;334:122233. doi: 10.1016/j.lfs.2023.122233. Epub 2023 Oct 31. Life Sci. 2023. PMID: 37918628 Review.
Cited by
-
Surface projections of titanium substrates increase antithrombotic endothelial function in response to shear stress.J Biomed Mater Res A. 2013 Nov;101(11):3181-91. doi: 10.1002/jbm.a.34613. Epub 2013 Apr 2. J Biomed Mater Res A. 2013. PMID: 23554161 Free PMC article.
-
Regulation of cell attachment, spreading, and migration by hydrogel substrates with independently tunable mesh size.Acta Biomater. 2022 Mar 15;141:178-189. doi: 10.1016/j.actbio.2022.01.025. Epub 2022 Jan 15. Acta Biomater. 2022. PMID: 35041902 Free PMC article.
-
Accelerating in situ endothelialisation of cardiovascular bypass grafts.Int J Mol Sci. 2014 Dec 29;16(1):597-627. doi: 10.3390/ijms16010597. Int J Mol Sci. 2014. PMID: 25551605 Free PMC article. Review.
-
Hypoxia Affects the Structure of Breast Cancer Cell-Derived Matrix to Support Angiogenic Responses of Endothelial Cells.J Carcinog Mutagen. 2013;Suppl 13:005. doi: 10.4172/2157-2518.S13-005. J Carcinog Mutagen. 2013. PMID: 24600535 Free PMC article.
-
Designer Hydrogels for Precision Control of Oxygen Tension and Mechanical Properties.J Mater Chem B. 2015 Oct 28;3(40):7939-7949. doi: 10.1039/C5TB01038A. Epub 2015 Aug 5. J Mater Chem B. 2015. PMID: 26693017 Free PMC article.
References
-
- van Obberghen-Schilling E, Tucker RP, Saupe F, Gasser I, Cseh B, Orend G. Fibronectin and tenascin-C: Accomplices in vascular morphogenesis during development and tumor growth. Int. J. of Dev. Biol. 2011;55(4-5):511–525. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. - PubMed
-
- Sun YL, Luo ZP, Fertala A, An KN. Direct quantification of the flexibility of type I collagen monomer. Biochem. Bioph. Res. Co. 2002;295(2):382–386. - PubMed
-
- Yadavalli VK, Svintradze DV, Pidaparti RM. Nanoscale measurements of the assembly of collagen to fibrils. Int. J. Biol. Macromol. 2010;46(4):458–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

