N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors
- PMID: 22389406
- PMCID: PMC3367832
- DOI: 10.1242/jcs.092726
N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors
Abstract
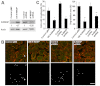
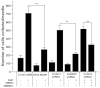
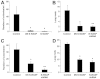
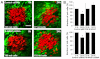
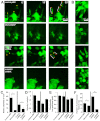
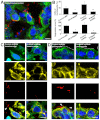
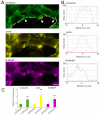
Invadopodia are proteolytic membrane protrusions formed by highly invasive cancer cells, commonly observed on substrate(s) mimicking extracellular matrix. Although invadopodia are proposed to have roles in cancer invasion and metastasis, direct evidence has not been available. We previously reported that neural Wiskott-Aldrich syndrome protein (N-WASP), a member of WASP family proteins that regulate reorganization of the actin cytoskeleton, is an essential component of invadopodia. Here, we report that N-WASP-mediated invadopodium formation is essential in breast cancer invasion, intravasation and lung metastasis. We established stable cell lines based on MTLn3 rat mammary adenocarcinoma cells that either overexpressed a dominant-negative (DN) N-WASP construct or in which N-WASP expression was silenced by a pSuper N-WASP shRNA. Both the N-WASP shRNA and DN N-WASP cells showed a markedly decreased ability to form invadopodia and degrade extracellular matrix. In addition, formation of invadopodia in primary tumors and collagen I degradation were reduced in the areas of invasion (collagen-rich areas in the invasive edge of the tumor) and in the areas of intravasation (blood-vessel-rich areas). Our results suggest that tumor cells in vivo that have a decreased activity of N-WASP also have a reduced ability to form invadopodia, migrate, invade, intravasate and disseminate to lung compared with tumor cells with parental N-WASP levels.
Figures
References
-
- Banzai Y., Miki H., Yamaguchi H., Takenawa T. (2000). Essential role of neural Wiskott-Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J. Biol. Chem. 275, 11987-11992 - PubMed
-
- Buccione R., Orth J. D., McNiven M. A. (2004). Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5, 647-657 - PubMed
-
- Chudakov D. M., Lukyanov S., Lukyanov K. A. (2007). Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat. Protoc. 2, 2024-2032 - PubMed
-
- Clark E. S., Whigham A. S., Yarbrough W. G., Weaver A. M. (2007). Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227-4235 - PubMed
-
- Condeelis J., Segall J. E. (2003). Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 3, 921-930 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

