Regulation of invadopodia formation and activity by CD147
- PMID: 22389410
- PMCID: PMC3367836
- DOI: 10.1242/jcs.097956
Regulation of invadopodia formation and activity by CD147
Abstract
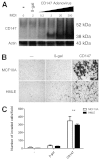
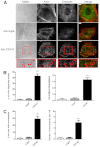
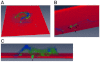
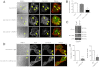
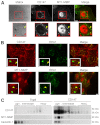
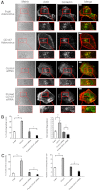
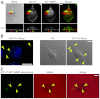
A defining feature of malignant tumor progression is cellular penetration through the basement membrane and interstitial matrices that separate various cellular compartments. Accumulating evidence supports the notion that invasive cells employ specialized structures termed invadopodia to breach these structural barriers. Invadopodia are actin-based, lipid-raft-enriched membrane protrusions containing membrane-type-1 matrix metalloproteinase (MT1-MMP; also known as matrix metalloproteinase 14; MMP14) and several signaling proteins. CD147 (emmprin, basigin), an immunoglobulin superfamily protein that is associated with tumor invasion and metastasis, induces the synthesis of various matrix metalloproteinases in many systems. In this study we show that upregulation of CD147 is sufficient to induce MT1-MMP expression, invasiveness and formation of invadopodia-like structures in non-transformed, non-invasive, breast epithelial cells. We also demonstrate that CD147 and MT1-MMP are in close proximity within these invadopodia-like structures and co-fractionate in membrane compartments with the properties of lipid rafts. Moreover, manipulation of CD147 levels in invasive breast carcinoma cells causes corresponding changes in MT1-MMP expression, invasiveness and invadopodia formation and activity. These findings indicate that CD147 regulates invadopodia formation and activity, probably through assembly of MT1-MMP-containing complexes within lipid-raft domains of the invadopodia.
Figures
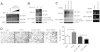
References
-
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034-3043 - PubMed
-
- Artym V. V., Yamada K. M., Mueller S. C. (2009). ECM degradation assays for analyzing local cell invasion. Methods Mol. Biol. 522, 211-219 - PubMed
-
- Berditchevski F., Chang S., Bodorova J., Hemler M. E. (1997). Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 272, 29174-29180 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

