TprC/D (Tp0117/131), a trimeric, pore-forming rare outer membrane protein of Treponema pallidum, has a bipartite domain structure
- PMID: 22389487
- PMCID: PMC3347077
- DOI: 10.1128/JB.00101-12
TprC/D (Tp0117/131), a trimeric, pore-forming rare outer membrane protein of Treponema pallidum, has a bipartite domain structure
Erratum in
- J Bacteriol. 2014 Sep;196(18):3360
Abstract
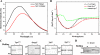
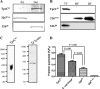
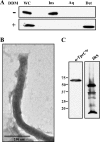
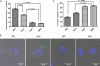
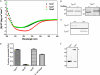
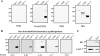
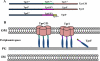
Identification of Treponema pallidum rare outer membrane proteins (OMPs) has been a longstanding objective of syphilis researchers. We recently developed a consensus computational framework that employs a battery of cellular localization and topological prediction tools to generate ranked clusters of candidate rare OMPs (D. L. Cox et al., Infect. Immun. 78:5178-5194, 2010). TP0117/TP0131 (TprC/D), a member of the T. pallidum repeat (Tpr) family, was a highly ranked candidate. Circular dichroism, heat modifiability by SDS-PAGE, Triton X-114 phase partitioning, and liposome incorporation confirmed that full-length, recombinant TprC (TprC(Fl)) forms a β-barrel capable of integrating into lipid bilayers. Moreover, TprC(Fl) increased efflux of terbium-dipicolinic acid complex from large unilamellar vesicles and migrated as a trimer by blue-native PAGE. We found that in T. pallidum, TprC is heat modifiable, trimeric, expressed in low abundance, and, based on proteinase K accessibility and opsonophagocytosis assays, surface exposed. From these collective data, we conclude that TprC is a bona fide rare OMP as well as a functional ortholog of Escherichia coli OmpF. We also discovered that TprC has a bipartite architecture consisting of a soluble N-terminal portion (TprC(N)), presumably periplasmic and bound directly or indirectly to peptidoglycan, and a C-terminal β-barrel (TprC(C)). Syphilitic rabbits generate antibodies exclusively against TprC(C), while secondary syphilis patients fail to mount a detectable antibody response against either domain. The syphilis spirochete appears to have resolved a fundamental dilemma arising from its extracellular lifestyle, namely, how to enhance OM permeability without increasing its vulnerability to the antibody-mediated defenses of its natural human host.
Figures

References
-
- Bredemeier R, et al. 2007. Functional and phylogenetic properties of the pore-forming beta-barrel transporters of the Omp85 family. J. Biol. Chem. 282:1882–1890 - PubMed
-
- Brusca JS, Radolf JD. 1994. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 228:182–193 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

