Metal transport across biomembranes: emerging models for a distinct chemistry
- PMID: 22389499
- PMCID: PMC3340188
- DOI: 10.1074/jbc.R111.319343
Metal transport across biomembranes: emerging models for a distinct chemistry
Abstract
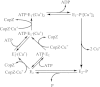
Transition metals are essential components of important biomolecules, and their homeostasis is central to many life processes. Transmembrane transporters are key elements controlling the distribution of metals in various compartments. However, due to their chemical properties, transition elements require transporters with different structural-functional characteristics from those of alkali and alkali earth ions. Emerging structural information and functional studies have revealed distinctive features of metal transport. Among these are the relevance of multifaceted events involving metal transfer among participating proteins, the importance of coordination geometry at transmembrane transport sites, and the presence of the largely irreversible steps associated with vectorial transport. Here, we discuss how these characteristics shape novel transition metal ion transport models.
Figures


Similar articles
-
Transition metal speciation in the cell: insights from the chemistry of metal ion receptors.Science. 2003 May 9;300(5621):931-6. doi: 10.1126/science.1085049. Science. 2003. PMID: 12738850 Review.
-
In vitro reconstitution of transition metal transporters.J Biol Chem. 2024 Aug;300(8):107589. doi: 10.1016/j.jbc.2024.107589. Epub 2024 Jul 19. J Biol Chem. 2024. PMID: 39032653 Free PMC article. Review.
-
Highlighting the roles of transition metals and speciation in chemical biology.Curr Opin Chem Biol. 2022 Aug;69:102155. doi: 10.1016/j.cbpa.2022.102155. Epub 2022 May 25. Curr Opin Chem Biol. 2022. PMID: 35643024 Review.
-
The thermodynamics of protein interactions with essential first row transition metals.Biochim Biophys Acta. 2016 May;1860(5):879-891. doi: 10.1016/j.bbagen.2015.11.005. Epub 2015 Nov 10. Biochim Biophys Acta. 2016. PMID: 26569121 Free PMC article. Review.
-
Interaction between transition metals and phenylalanine: a combined experimental and computational study.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Mar 5;138:499-508. doi: 10.1016/j.saa.2014.11.084. Epub 2014 Nov 29. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25528509
Cited by
-
Variable primary coordination environments of Cd(II) binding to three helix bundles provide a pathway for rapid metal exchange.Metallomics. 2015 Dec;7(12):1555-61. doi: 10.1039/c5mt00228a. Epub 2015 Oct 27. Metallomics. 2015. PMID: 26503746 Free PMC article.
-
Transporter excess and clustering facilitate adaptor protein shuttling for bacterial efflux.Cell Rep Phys Sci. 2025 Feb 19;6(2):102441. doi: 10.1016/j.xcrp.2025.102441. Epub 2025 Feb 12. Cell Rep Phys Sci. 2025. PMID: 40083904 Free PMC article.
-
XAS spectroscopy, sulfur, and the brew within blood cells from Ascidia ceratodes.J Inorg Biochem. 2014 Feb;131:99-108. doi: 10.1016/j.jinorgbio.2013.11.004. Epub 2013 Nov 16. J Inorg Biochem. 2014. PMID: 24333825 Free PMC article.
-
Molecular Dynamics Simulation of 2-Benzimidazolyl-Urea with DPPC Lipid Membrane and Comparison with a Copper(II) Complex Derivative.Membranes (Basel). 2021 Sep 28;11(10):743. doi: 10.3390/membranes11100743. Membranes (Basel). 2021. PMID: 34677508 Free PMC article.
-
Copper signalling: causes and consequences.Cell Commun Signal. 2018 Oct 22;16(1):71. doi: 10.1186/s12964-018-0277-3. Cell Commun Signal. 2018. PMID: 30348177 Free PMC article. Review.
References
-
- Fraústro da Silva J. J. R., Williams R. J. P. (2001) The Biological Chemistry of the Elements, 2nd Ed., Oxford University Press, New York
-
- Osman D., Cavet J. S. (2008) Copper homeostasis in bacteria. Adv. Appl. Microbiol. 65, 217–247 - PubMed
-
- Outten C. E., O'Halloran T. V. (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

