N-Ethylmaleimide Stimulates and Inhibits Ion Transport in Vestibular Dark Cells of Gerbil
- PMID: 22389574
- PMCID: PMC3291124
N-Ethylmaleimide Stimulates and Inhibits Ion Transport in Vestibular Dark Cells of Gerbil
Abstract
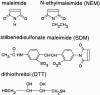
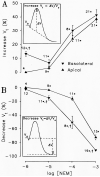
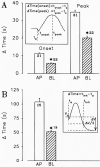
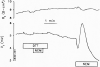
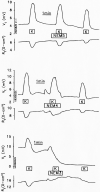
Vestibular dark cell epithelium was isolated from the semicircular canal of gerbils to test the proposal that the sulfhydryl alkylating agent N-ethylmaleimide (NEM) inhibits K(+) secretion by this tissue and does so by reacting with a site in or near the apical membrane. Dark cell epithelium was mounted in a micro-Ussing chamber for measurements of transepithelial voltage (V(t)) and resistance (R(t)) or in a perfused bath on the stage of a microscope for measurement of cell height as an index of cell volume. Perfusion of the apical or basolateral side with 10(-3) M NEM caused an increase in V(t) superimposed upon a slower decrease of V(t), resulting in a triphasic response. There were only small changes in R(t). Under this condition, V(t) is proportional to short circuit current and to K(+) secretion. Both the stimulatory and the inhibitory responses of V(t) were dose-dependent between 10(-6) and 10(-3) M NEM and the inhibition was irreversible. The specificity of the reaction of NEM with sulfhydryl groups was confirmed by the use of the reducing agent dithiothreitol (DTT). Perfusion of 5×10(-4) M DTT on the apical side caused no significant changes in V(t) but completely prevented both stimulation and inhibition of V(t) by NEM (10(-3) M). The amplitudes of the stimulation and the inhibition of V(t) were greater for basolateral than for apical perfusion of NEM. Similarly, the response times for each effect were faster from the basolateral side, suggesting that the primary sites of action are at or near the basolateral membrane. The site of action of NEM was further explored by subjecting the tissue to a membrane-impermeant sulfhydryl reagent, stilbenedisulfonate maleimide (SDM). Apical perfusion of 10(-3) M SDM had no effect on V(t) or R(t), whereas basolateral perfusion caused a reversible increase of V(t) (5.2 ± 0.5 to initially 6.8 ± 0.5 mV which relaxed after 60 s to 5.8 ± 0.5 mV) and to an initial decrease in R(t) by 4%. No inhibitory phase was observed. Elevation of basolateral [K(+)] from 3.6 to 25 mM is known to increase V(t) and reduce R(t) via direct stimulation of basolateral K(+) uptake and indirect stimulation of the apical membrane conductance. Basolateral perfusion of 10(-3) M NEM fully inhibited the increase of V(t) due to 25 mM K(+). Elevation of basolateral [K(+)] from 3.6 to 25 mM is known to increase reversibly cell volume. NEM was found to inhibit cell swelling in a dose-dependent manner but did not initially affect the rate of shrinking after K(+)-induced swelling, pointing to action only on basolateral transport pathways. The effects of NEM on K(+)-induced cell swelling were completely prevented by 5×10(-4) M DTT, demonstrating that the inhibitory effect of NEM was on sulfhydryl groups. In contrast to interpretations of NEM action in frog semicircular canal, we have found that NEM appears to stimulate an ion transport process in mammalian dark cells at an extracellular site in the basolateral membrane and inhibits another ion transport process in the basolateral membrane at another site. Inhibition by NEM from the apical side occurs most likely by diffusion of the agent to a site at or near the cytosolic side of the basolateral membrane.
Figures






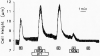
Similar articles
-
K(+)-induced stimulation of K+ secretion involves activation of the IsK channel in vestibular dark cells.Hear Res. 1996 Oct;100(1-2):201-10. doi: 10.1016/0378-5955(96)00127-x. Hear Res. 1996. PMID: 8922995
-
Evidence for Purinergic Receptors in Vestibular Dark Cell and Strial Marginal Cell Epithelia of Gerbil.Audit Neurosci. 1995;1(4):331-340. Audit Neurosci. 1995. PMID: 22582019 Free PMC article.
-
Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro.Hear Res. 1995 Apr;84(1-2):19-29. doi: 10.1016/0378-5955(95)00009-s. Hear Res. 1995. PMID: 7642451
-
Microelectrode and impedance analysis of anion secretion in Calu-3 cells.JOP. 2001 Jul;2(4 Suppl):219-28. JOP. 2001. PMID: 11875263 Review.
-
K+ and Cl- conductances in the distal colon of the rat.Gen Pharmacol. 1998 Sep;31(3):337-42. doi: 10.1016/s0306-3623(97)00458-8. Gen Pharmacol. 1998. PMID: 9703198 Review.
References
-
- Feng Y, Forgac M. Cysteine 254 of the 73-kDa A subunit is responsible for inhibition of the coated vesicle (H+)-ATPase upon modification by sulfhydryl reagents. J. Biol. Chem. 1992;267:5817–5822. - PubMed
-
- Fryer MW. An N-ethylmaleimide-sensitive G-protein modulates Aplysia Ca2+ channels. Neurosci. Lett. 1992;146:84–86. - PubMed
-
- George JN, Turner RJ. Inactivation of the rabbit parotid Na/K/Cl cotransporter by N-ethylmaleimide. J. Membr. Biol. 1989;112:51–58. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
