Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas
- PMID: 22389665
- PMCID: PMC3289615
- DOI: 10.1371/journal.pone.0030313
Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas
Abstract
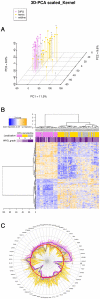
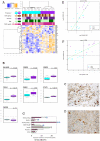
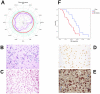
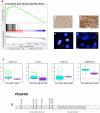
Diffuse intrinsic pontine glioma (DIPG) is one of the most frequent malignant pediatric brain tumor and its prognosis is universaly fatal. No significant improvement has been made in last thirty years over the standard treatment with radiotherapy. To address the paucity of understanding of DIPGs, we have carried out integrated molecular profiling of a large series of samples obtained with stereotactic biopsy at diagnosis. While chromosomal imbalances did not distinguish DIPG and supratentorial tumors on CGHarrays, gene expression profiling revealed clear differences between them, with brainstem gliomas resembling midline/thalamic tumours, indicating a closely-related origin. Two distinct subgroups of DIPG were identified. The first subgroup displayed mesenchymal and pro-angiogenic characteristics, with stem cell markers enrichment consistent with the possibility to grow tumor stem cells from these biopsies. The other subgroup displayed oligodendroglial features, and appeared largely driven by PDGFRA, in particular through amplification and/or novel missense mutations in the extracellular domain. Patients in this later group had a significantly worse outcome with an hazard ratio for early deaths, ie before 10 months, 8 fold greater that the ones in the other subgroup (p = 0.041, Cox regression model). The worse outcome of patients with the oligodendroglial type of tumors was confirmed on a series of 55 paraffin-embedded biopsy samples at diagnosis (median OS of 7.73 versus 12.37 months, p = 0.045, log-rank test). Two distinct transcriptional subclasses of DIPG with specific genomic alterations can be defined at diagnosis by oligodendroglial differentiation or mesenchymal transition, respectively. Classifying these tumors by signal transduction pathway activation and by mutation in pathway member genes may be particularily valuable for the development of targeted therapies.
Conflict of interest statement
Figures

References
-
- Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. The lancet oncology. 2006;7:241–248. - PubMed
-
- Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. - PubMed
-
- Herrington B, Kieran MW. Small molecule inhibitors in children with malignant gliomas. Pediatr Blood Cancer. 2009;53:312–317. - PubMed
-
- Jalali R, Raut N, Arora B, Gupta T, Dutta D, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2010;77:113–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

