Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones
- PMID: 22389696
- PMCID: PMC3289648
- DOI: 10.1371/journal.pone.0032366
Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones
Abstract
Neutrophils play an important role in innate immunity by defending the host organism against invading microorganisms. Antimicrobial activity of neutrophils is mediated by release of antimicrobial peptides, phagocytosis as well as formation of neutrophil extracellular traps (NET). These structures are composed of DNA, histones and granular proteins such as neutrophil elastase and myeloperoxidase. This study focused on the influence of NET on the host cell functions, particularly on human alveolar epithelial cells as the major cells responsible for gas exchange in the lung. Upon direct interaction with epithelial and endothelial cells, NET induced cytotoxic effects in a dose-dependent manner, and digestion of DNA in NET did not change NET-mediated cytotoxicity. Pre-incubation of NET with antibodies against histones, with polysialic acid or with myeloperoxidase inhibitor but not with elastase inhibitor reduced NET-mediated cytotoxicity, suggesting that histones and myeloperoxidase are responsible for NET-mediated cytotoxicity. Although activated protein C (APC) did decrease the histone-induced cytotoxicity in a purified system, it did not change NET-induced cytotoxicity, indicating that histone-dependent cytotoxicity of NET is protected against APC degradation. Moreover, in LPS-induced acute lung injury mouse model, NET formation was documented in the lung tissue as well as in the bronchoalveolar lavage fluid. These data reveal the important role of protein components in NET, particularly histones, which may lead to host cell cytotoxicity and may be involved in lung tissue destruction.
Conflict of interest statement
Figures
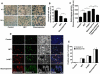
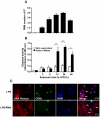
 SD), *p<0.05; **p<0.01; ***p<0.001. (D) Immunofluorescence staining of A549 cells after 16 h treatment with two concentrations of NET (3.4 and 10.1 µg/ml DNA-NET) or staurosporine was performed for ethidium homodimer III (ethidium-HD, red), annexin V (green), and Hoechst 33342 (black and white). Shown are representative pictures of three independent experiments. (E) Percentage of ethidium-HD and annexin-V positive cells from (D) was evaluated by morphometry analysis.
SD), *p<0.05; **p<0.01; ***p<0.001. (D) Immunofluorescence staining of A549 cells after 16 h treatment with two concentrations of NET (3.4 and 10.1 µg/ml DNA-NET) or staurosporine was performed for ethidium homodimer III (ethidium-HD, red), annexin V (green), and Hoechst 33342 (black and white). Shown are representative pictures of three independent experiments. (E) Percentage of ethidium-HD and annexin-V positive cells from (D) was evaluated by morphometry analysis.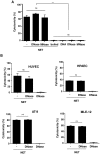
 SD), ***p<0.001, and ns = non-significant. (B) The degree of cytotoxicity was measured after treatment of HUVEC, HPAEC, AT-II or MLE-12 cells for 16 h with undigested NET (−) or completely (DNase) forms of NET as well as DNA alone. Shown are representative data of three (except for AT-II, n = 2) independent experiments (mean
SD), ***p<0.001, and ns = non-significant. (B) The degree of cytotoxicity was measured after treatment of HUVEC, HPAEC, AT-II or MLE-12 cells for 16 h with undigested NET (−) or completely (DNase) forms of NET as well as DNA alone. Shown are representative data of three (except for AT-II, n = 2) independent experiments (mean  SD), ns = non-significant.
SD), ns = non-significant.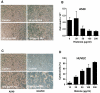
 SD), ***p<0.001 and ns = non-significant.
SD), ***p<0.001 and ns = non-significant.
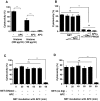
 SD), ***p<0.001 and ns = non-significant. (B) Histones or (C) NET were pre-incubated with antibody against histone H4 or polysialic acid (PSA), followed by incubation with A459 cells for 16 h to analyze the cytotoxicity. Note that polysialic acid considerably decreased both histone- and NET-mediated cytotoxicity.
SD), ***p<0.001 and ns = non-significant. (B) Histones or (C) NET were pre-incubated with antibody against histone H4 or polysialic acid (PSA), followed by incubation with A459 cells for 16 h to analyze the cytotoxicity. Note that polysialic acid considerably decreased both histone- and NET-mediated cytotoxicity.
 SD), ***p<0.001 and ns = non-significant.
SD), ***p<0.001 and ns = non-significant.
 SD), *p<0.05 and ns = non-significant.
SD), *p<0.05 and ns = non-significant.
 SD), ***p<0.001.
SD), ***p<0.001.References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

