How well do randomized trials inform decision making: systematic review using comparative effectiveness research measures on acupuncture for back pain
- PMID: 22389699
- PMCID: PMC3289651
- DOI: 10.1371/journal.pone.0032399
How well do randomized trials inform decision making: systematic review using comparative effectiveness research measures on acupuncture for back pain
Abstract
Background: For Comparative Effectiveness Research (CER) there is a need to develop scales for appraisal of available clinical research. Aims were to 1) test the feasibility of applying the pragmatic-explanatory continuum indicator summary tool and the six CER defining characteristics of the Institute of Medicine to RCTs of acupuncture for treatment of low back pain, and 2) evaluate the extent to which the evidence from these RCTs is relevant to clinical and health policy decision making.
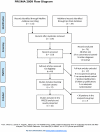
Methods: We searched Medline, the AcuTrials™ Database to February 2011 and reference lists and included full-report randomized trials in English that compared needle acupuncture with a conventional treatment in adults with non-specific acute and/or chronic low back pain and restricted to those with ≥30 patients in the acupuncture group. Papers were evaluated by 5 raters.
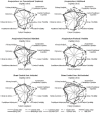
Principal findings: From 119 abstracts, 44 full-text publications were screened and 10 trials (4,901 patients) were evaluated. Due to missing information and initial difficulties in operationalizing the scoring items, the first scoring revealed inter-rater and inter-item variance (intraclass correlations 0.02-0.60), which improved after consensus discussions to 0.20-1.00. The 10 trials were found to cover the efficacy-effectiveness continuum; those with more flexible acupuncture and no placebo control scored closer to effectiveness.
Conclusion: Both instruments proved useful, but need further development. In addition, CONSORT guidelines for reporting pragmatic trials should be expanded. Most studies in this review already reflect the movement towards CER and similar approaches can be taken to evaluate comparative effectiveness relevance of RCTs for other treatments.
Conflict of interest statement
Figures
References
-
- Committee on Comparative Effectiveness Research PrioritizationInstitute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington D.C.: The National Academies Press; 2009. What is Comparative Effectiveness Research?29
-
- Conway PH, Clancy C. Comparative-Effectiveness Research – Implications of the Federal Coordinating Council's Report. N Engl J Med. 2009;361:330. - PubMed
-
- Last J, Spasoff RA, Harris S. A dictionary of epidemiology. Oxford: Oxford University Press; 2001.
-
- Committee on Comparative Effectiveness Research PrioritizationInstitute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington D.C.: The National Academies Press; 2009. Optimizing Evidence.31
-
- Schwarz D, Lelloch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637–647. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical